Antimicrobial stewardship programs have been defined as “coordinated interventions” designed to assess and enhance the appropriate use of antimicrobials by promoting the selection of the ideal antimicrobial drug regimen, dose, duration, and route of administration.1 These programs seek to achieve optimal clinical outcomes related to antimicrobial use and to minimize common adverse events. Antimicrobial stewardship programs have also been designed to reduce the costs of health care for management of infections and to limit antimicrobial selection for resistant microorganisms.1
Antimicrobial resistance (AMR) is considered a critical public health concern,2 and has become an international health threat. The financial burden and economic consequences are far beyond the health sector and extend to international travel and trade. Nosocomial infections across Europe have caused more than 25,000 deaths annually, and productivity losses and health care costs of at least €1.5 billion annually.2 Antimicrobial stewardship surveillance data from Europe and both American continents reported rising AMR across the microorganisms responsible for hospital-acquired and community-acquired infections. This has resulted in additional morbidity and mortality and has led to the use of newer and more costly antimicrobials; some of which are also associated with higher rates of adverse reactions.3,4 The situation in less developed countries is even more challenging. Data on antimicrobial use in India between 2005 and 2009 has showed increased consumption of all studied antimicrobial agents.5 The Antibiotic Resistance Surveillance and Control in the Mediterranean Region (ARMed) study collected data on antimicrobial use from hospitals in seven countries.6 It found a wide use of broad spectrum antimicrobial agents and a high prevalence of resistance in common pathogens.
The cornerstone of national and international efforts to control AMR is through the establishment of effective surveillance programs that track antimicrobial use among prescribers and monitor emergence and spread of resistant strains of microorganisms. Combined surveillance on both antimicrobial use and resistance has been shown to be beneficial and feasible in well-functioning health systems.2 Such surveillance programs help infectious disease experts to understand the relationship between antimicrobial consumption and AMR and enable them to build or change important policies to reduce rates of AMR.2 A meta-analysis on the prevalence of AMR in clinical isolates from Gulf Corporation Council (GCC) countries published in 2012 reported that most countries lacked guidelines for antimicrobial use and did not have policies for a proper auditing and restriction of antimicrobial prescriptions. Most GCC countries lack antimicrobial stewardship programs, especially in inpatient settings where broad-spectrum antimicrobial agents are widely used.7
In Oman, national guidelines for antimicrobial use have yet to be established. There are only three reports from Oman on the prevalence of AMR in some clinical isolates.7 The aim of our study was to assess and analyze the pattern and appropriateness of antimicrobial prescribing among physicians in acute care settings in a tertiary care hospital in Oman.
Methods
This retrospective study looked at antimicrobial prescribing data in patients admitted to acute care over a four week period (1–28 November 2012). The acute medical wards were comprised of four medical teams, and each had a senior internist and three residents. The average number of patients admitted per team was 30 over the study period. Patients were admitted for, on average, three to seven days. Patients’ data was retrieved from the Hospital Information System on a weekly basis by reviewing the records of discharged patients. Each infection was recorded as a single variable or episode. Patient data was entered in a manual form and included the following: diagnosis, site of infection, antimicrobial appropriateness (selection, route of administration, dosage, duration, frequency, and indication/s), allergy, and adverse events.
The manual forms of all patients who received antibiotics were reviewed and evaluated by an expert infectious disease consultant on a weekly basis. The rationality of antimicrobial use was evaluated, analyzed, and judged based on standard local and international guidelines, and the experience of the evaluating consultant who was blinded to the teams.
Descriptive statistics was used to describe the data. For categorical variables, frequencies and percentages were reported. For continuous variables, the mean and standard deviation (SD) were presented. For abnormally distributed variables, the median and interquartile range were used. Descriptive statistical analyses were done using Stata, (StataCorp, College Station, Texas, US) version 13.1.
Results
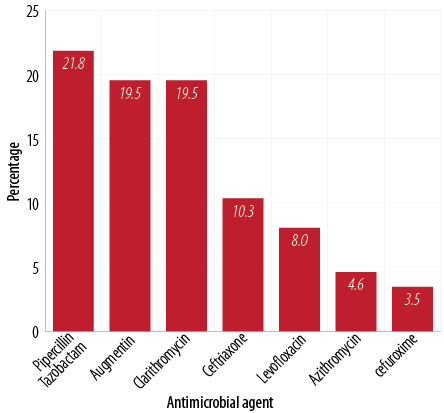
Figure 1: Commonly used antimicrobials.
Among the 178 patients discharged from acute medical wards over the study period, 114 patients (64%) received antimicrobial drugs during their admission. This translated into a total of 287 antimicrobial agents prescribed, with an average of 2.5±1.1 antimicrobial agents per patient. The most commonly prescribed agent was piperacillin/tazobactam followed by amoxicillin/clavulanic acid and clarithromycin [Figure 1]. The most common mode of administration was the intravenous route. Antimicrobial agents were prescribed mostly for community-acquired pneumonia (CAP) followed by urinary tract infection (UTI) as shown in Table 1.
Piperacillin/tazobactam was the most common antimicrobial agent prescribed for CAP, UTIs, wound infection, and aspiration pneumonia. The most common antimicrobial agent used for cellulitis was cloxacillin. Metronidazole was the most common agent used for gastroenteritis.
Cultures were obtained before antimicrobial initiation in 25% of infections. The most common isolated organisms were gram-negative bacteria [Table 2]. There was no organism isolated in 66% of the cultures. Antimicrobial agents were used empirically in 79% (n = 228) of infections while 21% (n = 59) of the infections were treated with antimicrobial agents based on available culture results. Antimicrobial agents were changed based on culture results in 12% of cases.
Table 1: Common infections for which antimicrobials were prescribed.
|
Community-acquired
pneumonia |
87 |
30.3 |
|
Urinary tract infection (UTI) |
40 |
13.9 |
|
No known focus |
28 |
9.8 |
|
Infected wound |
23 |
8.0 |
|
Gastroenteritis |
17 |
5.9 |
|
Bacteremia |
17 |
5.9 |
|
Aspiration pneumonia |
14 |
4.9 |
|
Cellulitis |
10 |
3.5 |
|
Catheter-related UTI |
9 |
3.1 |
Table 2: Commonly isolated organisms from different culture sites.
|
Polymicrobial |
23 |
8.0 |
|
Klebsiella sp. (ESBL Kleb) |
16 (1) |
5.6 |
|
Escherichia. coli (ESBL E.coli) |
16 (5) |
5.6 |
|
Pseudomonas. aeruginosa |
8 |
2.8 |
|
Acinetobacter sp. |
7 |
2.4 |
|
Enterobacter sp. |
7 |
2.4 |
|
Salmonella sp. |
5 |
1.7 |
|
Other gram negatives |
10 |
3.5 |
|
Candida albicans and other Candida sp. |
5 |
1.7 |
ESBL: extended spectrum beta-lactamase producers; sp.: species.
The prescribed antimicrobial agents were selected appropriately in 63% of infections. The median duration of antibiotics use was eight days (range = 6–11). The average duration of treatment for gram-negative organisms and gram-positive organisms was eight (range = 5–11) and 10.5 (range = 9–12) days, respectively. Figure 2 shows the average duration of antimicrobial use for the most common infections encountered.
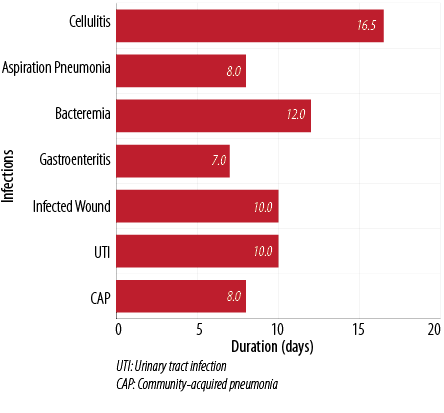
Figure 2: Average duration of antimicrobial use.
The dose and route of administration were appropriate in 66% and 70% of all prescribed antimicrobial agents, respectively, and the frequency was appropriate in 68% of all prescribed antimicrobial agents. There were no allergic reactions documented during the study period.
Discussion
In our study, a high proportion of patients (64%) received antimicrobial treatment while admitted in acute medical wards. Microbiological cultures were collected in only one-quarter of patients before the initiation of antimicrobial agents. Antimicrobials were prescribed empirically prior to the availability of culture results, and once the results were obtained the antimicrobials were modified in only 12% of patients.
This prescribing behavior was possibly related to the attitudes of health care professionals toward antimicrobial use in patients’ management. An antibiotic utilization study in Saudi Arabia, carried out in a tertiary care center over a 12–month period, also found similar prescribing behavior in terms of failure to modify antimicrobial agents based on available cultures results. Fifty-two positive blood cultures were found in 43 patients (1.2 infections per patient), and initial antimicrobial selection was appropriate in around 63% of all encountered antimicrobial agents.8 Antimicrobial modification based on available culture results was performed in only 12% of patients, which is comparable to the results found in our study.8
The most commonly prescribed antimicrobial agent was piperacillin/tazobactam, which is a broad-spectrum penicillin containing a β-lactamase inhibitor. The ARMed study showed that broad-spectrum penicillin, with or without a β-lactamase inhibitor, together with first-generation cephalosporin, were responsible for the bulk of antibiotics prescribed, and constituted at least one-third of the total usage in 15 of 25 hospitals in the Mediterranean region.7 Additionally, Safaeian et al,9 conducted a regular drug-use survey in 2010 on prescribing patterns in Isfahan province in Iran and found that penicillins were the most frequently prescribed antibiotics including amoxicillin (5.9%) and penicillin G benzathine.
A prospective study in 2008, conducted in the medical wards of a tertiary care hospital in Malaysia, found that co-amoxiclav, erythromycin, cefuroxime, and ceftriaxone, and ampicillin with sulbactam were the most commonly used antimicrobial agents.10 Variability in prescribing antimicrobial agents for common infections has been observed in several studies. The national antimicrobial utilization surveillance program 2006–2007 annual report showed that there was a great variability in the commonly prescribed antibiotics among countries in Europe compared to Australia.11
Cultures were not obtained prior to antimicrobial initiation in three-quarters of infection episodes. Culture collection prior to initiation of antimicrobial agents was influenced by many factors especially the attitude of physicians and nurses toward the importance of obtaining cultures prior to starting antibiotics as well as the patient’s condition (such as refusal and illness severity). Al Shimemeri et al,8 found that 72% of patients had received antibiotics before or soon after obtaining the blood culture. In 2012, Aly and Balkhy7 conducted a study on the prevalence of antimicrobial resistance in clinical isolates from several Gulf Corporation Council (GCC) countries. The most prevalent microorganism was Escherichia coli, followed by Klebsiella pneumoniae, Pseudomonas aeruginosa, methicillin-resistant Staphylococcus aureus (MRSA), and Acinetobacter. Al Shimemeri et al,8 found that the most commonly isolated organisms in their institution were gram-positive cocci (60%). This difference could be influenced by the types of infections seen in each study. In our study, most of the patients had infections from gram-negative organisms, such as K. pneumoniae and E. coli. Higher rates of extended spectrum beta-lactamase producers (ESBL) organisms were observed. [Table 2].
The high prevalence of resistant gram-negative isolates has been attributed to several factors including easy availability of broad-spectrum antibiotics, lack of antimicrobial stewardship programs, old architectural design preventing proper isolation of infected or colonized patients, lack of strong infection control programs, and a lack of well-trained specialists and clinical pharmacists in infectious disease field.7
In 1990, Aswapokee et al,12 conducted a study in nine medical wards in one of the hospitals in Thailand. The authors reported that 44% of admitted patients had been prescribed antibiotics, but only 8% received an appropriate antimicrobial agent. Antibiotics were used without evidence of infection in 35% of the patients. A survey of inpatient antibiotic use in a teaching hospital in South Africa in the medical, surgical, and gynecological wards showed that 27–32% of patients were prescribed antibiotics, and 22% of antibiotics were used inappropriately.13 In our study, the antimicrobial agents were prescribed appropriately in 63% of infections, which can be explained by the availability of infectious disease consultation during admission. However, the small sample size from a single tertiary care institution might not fully represent the overall prescribing patterns among physicians in other tertiary care institutions in Oman.
Our study has several limitations. Being a retrospective study that included only a small number of patients from a single tertiary care hospital, caution is necessary for generalizing the results to the population at large. We analyzed antimicrobial utilization patterns over four weeks; however, a different pattern could have been observed if the study period was extended to cover other months or seasons. In addition, no grade of illness severity was included. For this reason, it was not possible to correlate the drug utilization patterns with the severity of patients’ illnesses. The data from this study was reviewed by a single infectious disease consultant, which might lead to bias due to possible differences among various experts in assessing the appropriateness of antimicrobial selection and misuse. Some patients had more than one infection in each admission or in the same admission, which may have resulted in a duplication of a variable or an episode. This can inflate the percentage of misuse. The data obtained from such patients with possible duplicated infections may contribute to a crossover effect on final frequency analysis. Despite these limitations, the data obtained from our study is still beneficial and should highlight the impact of common prescribing patterns among physicians in tertiary care hospitals in Oman that are similar to physicians prescribing patterns in developed and other developing countries.
Conclusion
This was the first study conducted in Oman addressing the patterns of antibiotic prescribing among internists in a tertiary care hospital. The study identified a need for national guidelines for the management of common infections to reduce overuse and misuse of antimicrobial agents in tertiary care hospitals in Oman. A large surveillance multi-center study in different hospitals areas or specialties on antimicrobial prescribing appropriateness is warranted.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study. An abstract of this research was published in a supplement of the January issue of the Oman Medical Journal 2014; 29(2): 148–163.
Acknowledgements
The authors would like to thank pharmacist Zaher Al-Salmi for his contribution to this study.
references
- 1. Infectious disease society of America. Arlington: Promoting Antimicrobial Stewardship in Human Medicine. Available at: http://www.idsociety.org. Accessed May 3, 2015.
- 2. Aaerestrup FM, Kane AA, Cars O, Heddini A, Chang S, So A, et al. The evolving threat of antimicrobial resistance Options for action (Internet). Geneva: world health organization; 2012. Available at http://www.who.int. Accessed May 3, 2015.
- 3. European Centre for Disease Prevention and Control. Stockholm: ECDC; 2010. Antimicrobial resistance surveillance in Europe 2009. Annual Report of the European Antimicrobial Resistance Surveillance Network (EARS-Net). Available at: http://ecdc.europa.eu. Accessed May 3, 2015.
- 4. Pan American Health Organization. Washington: PAHO; 2009. 4 Annual Report of the Monitoring / Surveillance of Resistance to Antibiotics. Available at: http://new.paho.org. Accessed May 3, 2015.
- 5. Ganguly NK, Wattal C, Chandy SJ, Arora SK, Gupta U, Kotwani A, et al. Situation Analysis Antibiotic Use and Resistance in India. Public Health Foundation of India, and Center for Disease Dynamics, Economics and Policy; 2011. Available at: http://www.cddep.org. Accessed May 3, 2015.
- 6. Borg MA, Zarb P, Ferech M, Goossens H; ARMed Project Group. Antibiotic consumption in southern and eastern Mediterranean hospitals: results from the ARMed project. J Antimicrob Chemother 2008 Oct;62(4):830–836.
- 7. Aly M, Balkhy HH. The prevalence of antimicrobial resistance in clinical isolates from Gulf Corporation Council countries. Antimicrob Resist Infect Control 2012;1(1):26.
- 8. Al Shimemeri A, Al Ghadeer H, Memish Z. Antibiotic utilization pattern in a general medical ward of a tertiary medical center in Saudi Arabia. Avicenna J Med 2011 Jul;1(1):8–11.
- 9. Safaeian L, Mahdanian AR, Hashemi-Fesharaki M, Salami S, Kebriaee-Zadeh J, Sadeghian GH. General physicians and prescribing pattern in isfahan, iran. Oman Med J 2011 May;26(3):205–206.
- 10. Akter SF, Rani MF, Rahman JA, Nordin MS, Satwi S, Awang MB. Antimicrobial use and factors influencing prescribing in medical wards of a tertiary care hospital in Malaysia. Int J Sci Environ Technol 2012;1(4):274–284.
- 11. McKenzie D, Rawlins M, Del Mar C. Antimicrobial stewardship: what’s it all about? Australian Prescriber 2013;36(4):116–120.
- 12. Aswapokee N, Vaithayapichet S, Heller RF. Pattern of antibiotic use in medical wards of a university hospital, Bangkok, Thailand. Rev Infect Dis 1990 Jan-Feb;12(1):136–141.
- 13. Till B, Williams L, Oliver SP, Pillans PI. A survey of inpatient antibiotic use in a teaching hospital. S Afr Med J 2009;6:80(1):7–10.