Prostate cancer is the second most common malignancy in men globally.1 Absence of the basal cell layer in prostate biopsy specimens along with the presence of abnormal mitosis is the standard criteria for reporting prostate adenocarcinoma.2 The major proportions of prostate carcinomas are acinar adenocarcinoma, although several rare histologic variants coexists.3
Ductal carcinoma is the most frequent of the rare histologic subtypes of prostate carcinoma, accounting for 5% of total prostate carcinoma cases and occurring most often in elderly men.3 Ductal tumors mainly arise from primary periurethral prostatic ducts whereas acinar tumors arise from other periurethral prostatic ducts.4 Gross hematuria and urinary obstruction are the primary complaints in patients with prostate adenocarcinomas. Both tumors present with an elevated serum prostate-specific antigen (PSA) levels.4
The majority of ductal adenocarcinomas are found in association with an acinar component. Tumors with absolute ductal components are extremely rare.3 The diagnosis of ductal and acinar adenocarcinoma generally depends on histopathologic and immunohistochemical examination.5 Histologically, ductal adenocarcinomas are composed of columnar cells arranged in either a papillary or cribriform pattern, whereas acinar adenocarcinomas exhibit cuboidal cells arranged in acini. The papillary pattern of ductal tumors consist of a true papillary fronds lined by columnar cells exhibiting a variable degree of nuclear pleomorphism and hyperchromasia. The other pattern consists of proliferating large, back-to-back cribriform glands with central necrosis. In both patterns, the surrounding stroma is fibrotic or altered.3,6 Based on the morphological features the grading of acinar component varies; however, the ductal component is usually graded as four on the Gleason scoring system.7
Adenocarcinoma of the prostate mainly express immunohistochemical markers of prostatic tissue, including PSA, prostatic acid phosphatase (PAP), alpha-methylacyl coenzyme A (CoA)-reductase (AMACR), androgen receptor (AR), and cytokeratin 7 (CK7). Basal cell markers, such as proto-oncogene (p63), high-molecular-weight CK (clone 34βE12) and CK5/6, are usually negative.4,5
The use of the 34βE12/p63/AMACR antibody cocktail has shown promising results in differentiating malignant prostatic glands from benign glands adjacent to cancer in a biopsy specimen.5 Furthermore, the presence of a ductal component in the tumor is reported to be associated with an aggressive disease, although the impact of the ductal component on the overall Gleason score and PSA levels remains controversial.8,9 Therefore, we investigated the use of the 34βE12/p63/AMACR antibody cocktail in the diagnosis of prostate adenocarcinoma and compared the association of ductal and acinar adenocarcinoma with clinicopathological variables such as patient’s age at diagnosis, cumulative Gleason score, and PSA levels.
Methods
A total of 150 transurethral resection of prostate (TURP) biopsy specimens of prostatic adenocarcinoma were obtained along with relevant clinical history and PSA levels from the surgical pathology files at the Dow Diagnostic Research and Reference Laboratory, Karachi. Diagnosis of prostate adenocarcinoma was confirmed by evaluating the morphological features and Gleason scores. The characterization of tumors into ductal and acinar cases and calculation of the percentage of the ductal component was determined by examination of multiple levels of hematoxylin and eosin (H&E) stained sections by a panel of consultant histopathologists. This study was conducted with institutional review board approval.
For immunohistochemistry, the method described by Jiang et al,5 was used. Briefly, formalin-fixed, paraffin-embedded tissue blocks were cut into 3μm sections, dipped into a hot water bath and transferred to glass slide followed by treatment with 0.1mol/L citrate buffer (pH 6.0) in a microwave for 15 minutes for antigen retrieval. A cocktail of the three antibodies was prepared: a mouse monoclonal antibody (34βE12, Dako) at a dilution of 1:50 was mixed with mouse monoclonal antibody, p63 (NeoMarkers, Fremont, CA) and rabbit monoclonal antibody AMACR (P504S, Corixa, Seattle, WA), each at a 0.5μg/mL dilution. The cocktail was then applied to the tissue sections for 45 minutes followed by a buffer rinse. The enzyme activity was developed separately. For double color reaction diaminobenzidine was applied for five minutes and fast red was applied for 20 minutes. The sections were counterstained with hematoxylin for 10–60 seconds, rinsed with distilled water, and dried at room temperature. The slides were then cover slipped with permanent mounting media. Immunohistochemistry slides (Fremont, CA, USA) were used as positive controls, while tissue slides incubated with tris-buffered saline (TBS) without primary antibodies served as negative controls.
The immunohistochemistry sections were examined by the same panel of consultant histopathologists. The positive AMACR staining was described by continuous, dark red cytoplasmic staining or apical granular staining patterns in the malignant epithelial cells that could be observed easily at low-power magnification (<100×), whereas 34βE12 and p63 were considered positive by dark brown cytoplasmic and nuclear staining in the basal cells respectively.
Statistical analysis was performed by SPSS version 21.0 (SPSS Inc., Chicago, USA). The clinicopathological parameters were divided into three groups; age (patients aged ≤65 years and those >65 years old), pathological tumor grade (Gleason’s scores ≤7 and >7), and PSA level (≤10ng/ml and >10ng/ml). The correlation between categorical variables with prostatic ductal and acinar adenocarcinoma was evaluated by binary logistic regression. In all tests, a minimum limit of significance was determined as p<0.050.
Results
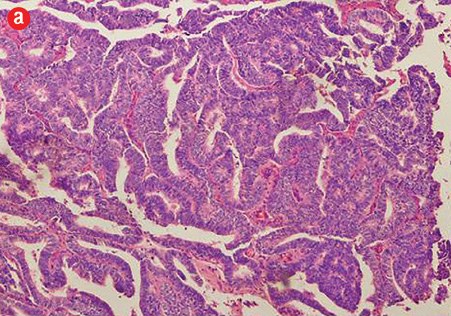
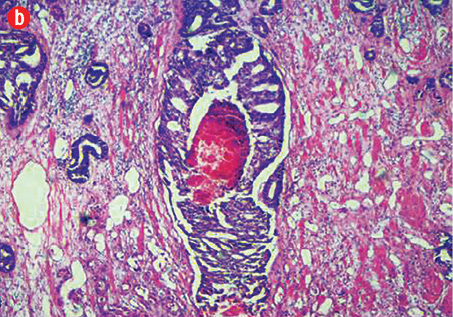
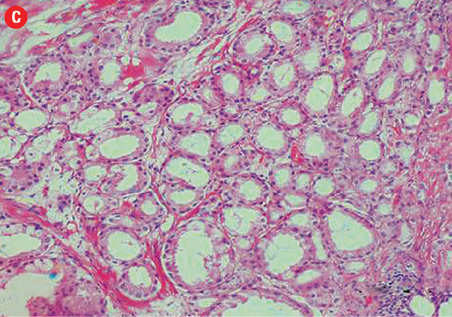
Based on morphological features, 10 out of 150 cases (7%) were characterized as ductal adenocarcinoma and 140 cases (93%) exhibited the morphological features of acinar adenocarcinoma. Ductal carcinoma cases were characterized by distinctive pseudostratified columnar epithelial cells in papillary or cribriform architectures, whereas acinar tumors exhibited glands and acini lined by a single layer of cuboidal cells [Figure 1]. The ductal component coexisted with conventional acinar adenocarcinoma in all ductal cases. A variable degree of mitosis was also present in a vast number of both tumors.
|
|
|
|
|
|
|
|
|
Figure 1: (a) Prostatic ductal adenocarcinoma with papillary morphology, 10× magnification.
(b) Cribriform prostatic ductal adenocarcinoma with central necrosis, 40× magnification.
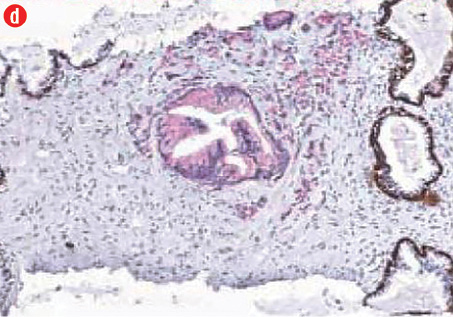
(c) Acinar adenocarcinoma exhibiting small round crowded glands lined by a single layer of cuboidal cells, 40× magnification. (d) Ductal adenocarcinoma with a red cytoplasmic granular staining pattern of AMACR and basal cells with dark brown nuclear (p63) and cytoplasmic (34βE12) staining in adjacent benign glands in the same slides, 40× magnification. |
Table 1: Expression of AMACR and basal cell markers (34βE12/p63) in prostatic carcinomas.
|
Malignant glands (prostate carcinoma) |
149 |
0 |
|
Benign glands |
0 |
150 |
|
adjacent to cancer |
|
|
All prostatic carcinoma showed a red cytoplasmic granular staining pattern of AMACR in the malignant glands and cells, and dark brown nuclear (p63) and cytoplasmic (34βE12) staining in basal cells in the adjacent nonmalignant glands. The immunohistochemical analysis and antibody expression are presented in Table 1 and Figure 1, respectively. Gross hematuria and urinary obstruction were the chief complaints in all cases.
Marked elevation in serum PSA levels (>10ng/ml) was observed in vast number of both subtypes. Unlike acinar tumors, the majority of patients with ductal cases (6 of 10) were aged ≤65 years old at the time of diagnoses. Overall, there were no significant differences except that most ductal adenocarcinomas (9 of 10) were significantly aggressive (p<0.030; Gleason grade >7). The clinicopathological characteristics and statistical estimates of all cases are summarized in Table 2.
Table 2: Clinicopathological comparison of ductal prostatic adenocarcinoma and acinar prostatic adenocarcinoma with statistical estimates.
|
Ductal (n=10) |
6 |
4 |
0.530* |
1 |
9 |
0.030* |
3 |
7 |
0.220* |
ap-values for comparisons between age and tumors; bp-values for comparisons between Gleason score and tumors; cp-values for comparisons between PSA level and tumors;*binary logistic regression test.
Discussion
Prostate cancer is the second most commonly diagnosed cancer worldwide, with a relatively high number of tumors being acinar in nature, although ductal adenocarcinoma, a rare variant of prostate adenocarcinoma coexists.3,4 In the present study, we assessed 150 tumors bearing TURP prostate biopsies and documented 10 ductal and 140 acinar subtype of prostatic adenocarcinoma. Ductal cases accounted for 7% of the total prostate adenocarcinomas, whereas the remaining 93% were acinar tumors. These rates were in line with the literature.10-12
Melicow et al,13 first characterized ductal adenocarcinoma as a rare variant which lacks the typical characteristics of acinar prostate adenocarcinomas in the early stages, such as elevated PSA and palpation induration. The atypical presentation of prostatic ductal adenocarcinoma meant that the condition remained unnoticed and, therefore, untreated. Due to its histologic resemblance and clinicopathological features of uterine endometrial carcinoma, prostatic ductal adenocarcinoma was initially termed “endometrioid carcinoma”. However, recently, it was renamed to “ductal carcinoma with endometrioid features” and then to “prostatic ductal adenocarcinoma”.4
The characterization of ductal and acinar adenocarcinomas is challenging, because both tumor types frequently coexist. In addition, ductal adenocarcinomas develop from the prostatic utricle, whereas acinar tumors arise from the urothelial tract, which further complicates diagnosis.4 However, with recent advances in diagnostic modalities and careful pathological analysis, it may become possible to differentiate prostatic ductal adenocarcinomas from the acinar subtype.
Previous studies have shown that H&E accompanied with immunohistochemistry using AMACR in combination with 34βE12 and p63 has 80% to 100% sensitivity for detecting prostate adenocarcinomas particularly in needle biopsy. Furthermore, immunohistochemical analysis with a triple-antibody cocktail is an assay with high specificity for prostate carcinoma and aids in differentiation of prostate cancer from high-grade prostatic intraepithelial neoplasia (PIN) and some benign lesions. The malignant diagnosis was established, when atypical glands identified by routine H&E staining were negative for basal cell markers (34βE12 and p63) and positive for AMACR (P504S).4,5
All patients in our study were elderly males and presented with gross hematuria as the chief clinical symptom. Our study demonstrated that characterization of prostatic ductal and acinar adenocarcinoma relied mainly on pathological and immunohistochemical examination. Papillary and cribriform architectures were the two predominant morphological patterns in the ductal subtype, in contrast with a predominant glandular and acinar pattern in acinar cases. Similar patterns were observed in previous investigations.2,6 Additionally, like previous studies, we determined that cancers with ductal histology are generally found to have an acinar component as well.3,14
In agreement with previous studies,4,5 our research showed that AMACR in combination with 34βE12 and p63 could be useful in distinguishing malignant prostatic glands from other benign glands adjacent to cancer and provides the opportunity to characterize suspicious areas on prostate biopsies. In addition, this triple-antibody analysis using automated immunostainer solutions is quick and reliable and, therefore, can be used in routine clinical practice.
The aggressive nature of prostatic adenocarcinoma accompanied with any amount of ductal histology was reported in recent literature.8,15 Additionally, Morgan et al,14 reported a significant relationship between ductal carcinoma with advanced age and increased risk of mortality. We determined that the tumor grade was significantly higher in ductal cases (p=0.030). This finding is in line with other recent studies.8,15 We therefore suggest that ductal carcinomas are more likely to be poorly differentiated and have metastatic disease than the acinar counterpart. However, contrary to Morgan et al,14 we reported that the majority of ductal cases were patients aged <65 years old with no statistically significant differences observed. The limited number of ductal cases included in our study could explain this discrepancy.
In previous studies, low PSA levels were seen in the initial stages of prostate ductal adenocarcinoma compared to acinar tumor. PSA levels begin to rise when the tumor becomes aggressive (with increasing Gleason score) and invades surrounding structures.4,16 Therefore, the possibility of prostatic ductal adenocarcinoma should still be considered when patients present without prostate nodules or an elevated PSA.4 In our study, marked elevation in the serum PSA level (>10ng/ml) in most ductal and acinar tumors was observed. This observed difference is most likely due to the vast number of patients with high-grade tumors enrolled in the study. Therefore, we recommend further investigation using a larger number of patients with well to moderately differentiated ductal carcinoma to confirm the link with serum PSA level.
There were several limitations in this study. Firstly, the cases analyzed were selected from those who underwent TURP and, therefore, patients who had undergone radical prostatectomy who had more advanced adenocarcinoma were not included. Secondly, needle biopsy specimens and other prostate lesions such as PIN were not included in this study and we were unable to determine the utility of triple antibody cocktail in the diagnosis of small focal prostate cancer and for differentiating prostate cancer from other non-neoplastic lesions. Thirdly, the use of TURP specimens limits us in considering other parameters, such as tumor stage, for comparative analyses. Finally, the cases of ductal carcinoma were limited in number and all ductal cases we examined showed more than 60% of the ductal component, thus we could not determine whether cases containing a lesser volume of ductal component showed more advanced pathological features compared to acinar adenocarcinoma.
Conclusion
In this study we characterized two important subtypes of prostate cancer. Our findings suggest that the diagnosis of ductal and acinar adenocarcinoma of the prostate can be based on pathological and immunohistochemical examinations. Ductal adenocarcinomas are more likely to present with a high pathological grade. Our findings suggest a more aggressive natural history of ductal adenocarcinomas when compared to acinar adenocarcinomas. Further prospective research is required to confirm our observations and would be helpful to identify the factors responsible for the differences between ductal and acinar prostate cancers.
Disclosure
The authors declared no conflicts of interest.
Acknowledgements
We thank Dr. Nadeem Khan, Mr. Manzoor Ahmed Asi and the histopathology team for their professional assistance when needed. Special thanks to vice chancellor, Prof. Dr. Masood Hameed Khan and all members of BASR, Dow University of Health Sciences for approving this research.
reference
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011 Mar-Apr;61(2):69-90.
- Humphrey PA. Histological variants of prostatic carcinoma and their significance. Histopathology 2012 Jan;60(1):59-74.
- Huang H, Chen F. Prostatic ductal adenocarcinoma exhibits more advanced histopathological features than acinar adenocarcinoma. N A J Med Sci 2012 Oct;5:208-211.
- Sha J, Bo J, Pan J, Zhang L, Xuan H, Chen W, et al. Ductal adenocarcinoma of the prostate: immunohistochemical findings and clinical significance. Onco Targets Ther 2013 Oct;6:1501-1506.
- Jiang Z, Li C, Fischer A, Dresser K, Woda BA. Using an AMACR (P504S)/34betaE12/p63 cocktail for the detection of small focal prostate carcinoma in needle biopsy specimens. Am J Clin Pathol 2005 Feb;123(2):231-236.
- Grignon DJ. Unusual subtypes of prostate cancer. Mod Pathol 2004 Mar;17(3):316-327.
- Delahunt B, Miller RJ, Srigley JR, Evans AJ, Samaratunga H. Gleason grading: past, present and future. Histopathology 2012 Jan;60(1):75-86.
- Samaratunga H, Duffy D, Yaxley J, Delahunt B. Any proportion of ductal adenocarcinoma in radical prostatectomy specimens predicts extraprostatic extension. Hum Pathol 2010 Feb;41(2):281-285.
- Amin A, Epstein JI. Pathologic stage of prostatic ductal adenocarcinoma at radical prostatectomy: effect of percentage of the ductal component and associated grade of acinar adenocarcinoma. Am J Surg Pathol 2011 Apr;35(4):615-619.
- Epstein JI, Woodruff JM. Adenocarcinoma of the prostate with endometrioid features. A light microscopic and immunohistochemical study of ten cases. Cancer 1986 Jan;57(1):111-119.
- Bostwick DG, Kindrachuk RW, Rouse RV. Prostatic adenocarcinoma with endometrioid features. Clinical, pathologic, and ultrastructural findings. Am J Surg Pathol 1985 Aug;9(8):595-609.
- Greene LF, Farrow GM, Ravits JM, Tomera FM. Prostatic adenocarcinoma of ductal origin. J Urol 1979 Mar;121(3):303-305.
- Melicow MM, Pachter MR. Endometrial carcinoma of proxtatic utricle (uterus masculinus). Cancer 1967 Oct;20(10):1715-1722.
- Morgan TM, Welty CJ, Vakar-Lopez F, Lin DW, Wright JL. Ductal adenocarcinoma of the prostate: increased mortality risk and decreased serum prostate specific antigen. J Urol 2010 Dec;184(6):2303-2307.
- Aydin H, Zhang J, Samaratunga H, Tan N, Magi-Galluzzi C, Klein E, et al. Ductal adenocarcinoma of the prostate diagnosed on transurethral biopsy or resection is not always indicative of aggressive disease: implications for clinical management. BJU Int 2010 Feb;105(4):476-480.
- Oxley JD, Abbott CD, Gillatt DA, MacIver AG. Ductal carcinomas of the prostate: a clinico-pathological and immunohistochemical study. Br J Urol 1998 Jan;81(1):109-115.