Acute coronary syndrome (ACS) is a term used for the clinical presentation of unstable angina pectoris, ST-elevation myocardial infarction, and non-ST elevation myocardial infarction caused due to myocardial ischemia because of disruption of atherosclerotic plaque linked with thrombotic vessel occlusion.1 The data from preclinical and clinical studies indicate that an inflammatory response is involved in all stages of atherosclerosis and it is considered a vital factor that plays a significant role in the subsequent onset of ACS, plaque instability, and further disease progression.2–4 Conventional methods to predict cardiac disease are limited to known risk factors such as age, hypertension, hyperlipidemia, diabetes, and tobacco and alcohol consumption, but a more comprehensive understanding of the mechanism can result in better risk stratification and targeted therapeutic therapy for coronary artery disease.5 During the past few decades, different pathways of inflammatory biomarkers, including the role of pro-inflammatory cytokines interleukin (IL)-1, IL-6, and IL-18 in patients with cardiovascular diseases (CVD) had been studied that can be useful for the early detection of the disease.6 Members of the IL-1 family such as IL-1 and IL-18, are well-known pro-inflammatory cytokines, and they contribute to the atherosclerotic process and onset of ACS.7 Research data from animal studies have revealed that IL-1β is critically involved in overall pro-atherogenic actions.8 However, IL-10 is an anti-inflammatory cytokine that exerts a protective action by reducing excessive inflammatory reactions. IL-10 is produced by various inflammatory cells including macrophages and it inhibits the synthesis of inflammatory cytokines.9 An imbalance between these anti-inflammatory and pro-inflammatory cytokines leads to CVD.10
Studies measuring serum levels of these ILs in patients with ACSs have generated conflicting results. A Mendelian randomization study found no association between IL-1β and IL-18 levels and the risk of CVD.11 On the contrary,1 it has been demonstrated that plasma IL-18 levels are associated with ACS due to significantly higher levels in ACS patients compared to healthy controls.
The primary objective of this study was to strengthen the concept that IL cytokines might reflect the status of systemic inflammation associated with ACS and potentially contribute to myocardial damage through the local release of pro-inflammatory molecules. The secondary endpoint is to determine the correlation of these inflammatory biomarkers with each other and the risk factors.
Methods
The research was designed as a case-control observational study to provide the accurate values of plasma biomarkers IL-6, IL-18, IL-1β, and IL-10 in the interventional group (ACS patients) and control group (healthy individuals) along with the correlation of these biomarkers with each other. The study was conducted at Hassan Sadikin Hospital, Bandung, Indonesia from September to December 2021. The clinical research study protocol was approved by the Research Ethics Committee Universitas Padjadjaran Bandung (Protocol number: 668/ UN6.KEP/EC/2021). All subjects gave informed consent, both verbal and written, for participation in the study.
A total of 43 participants from both genders over the age of 18 years were enrolled by purposive sampling technique. Subjects were divided into two groups: group I, ACS patients (n = 23), and group II, control group (n = 20). The blood samples were collected as soon as the patient was brought to the intensive care unit. Once a patient was diagnosed with ACS based on the typical clinical history of ACS (unstable angina pectoris, ST-elevation myocardial infarction, and non-ST elevation myocardial infarction), plus electrocardiographic data and cardiac enzymes at admission, the sample was included in the study. The selection was made sequentially, according to the inclusion criteria. The exclusion criteria for group I was if patients had a previous history of ACS, autoimmune disease, liver or end-stage kidney disease with an estimated glomerular filtration rate of ≤ 30 mL/min/1.73 m2, terminal illness, and those who did not sign the informed consent. However, healthy controls were included in group II if they had no more than one risk factor of CVD (i.e., smoking, alcohol use, hypertension, hyperlipidemia, diabetes, and obesity).
An 8 mL blood sample was taken from the vein of each participant in two sample collection tubes (4 mL) filled with heparin. Sample collection tubes were then labeled with the participant’s name. The blood was immediately centrifuged at room temperature within 30 minutes at 1500 rpm for 15 minutes. After centrifugation, two 1 mL aliquots of plasma were removed and stored at -80 °C.
Plasma levels of ILs were measured by quantitative sandwich enzyme immunoassay technique using a commercially available enzyme-linked immunosorbent assay kit (Sigma Aldrich): cytokines IL-6, IL-18, IL-1β, and IL-10, with sensitivities of 3 pg/mL, 0.5 pg/mL, 1 pg/mL, and 0.3 pg/mL, respectively.
The data were analyzed using the SPSS (IBM Corp. Released 2019. IBM SPSS Statistics for Windows, Version 26.0. Armonk, NY: IBM Corp). Discrete variables are expressed as counts (number and percentage) and continuous variables are expressed as mean±SD and compared using the independent sample t-test. Spearman’s correlation test was done to check the correlation between variables. A p-value of <0.05 was considered statistically significant and the confidence level was 95%.
Results
The demographic characteristics of the patients are summarized in Table 1.
Clinical characteristics and laboratory measurements of the patients are summarized in Table 2. There was a significant difference in mean values of age, systolic blood pressure, diastolic blood pressure, and body mass index in the ACS and control group (p < 0.05). The other parameters showed no significant difference between the two groups (i.e., total cholesterol and fasting blood sugar).
The mean concentration of IL-6 (298.6±432.9 pg/mL) in ACS patients was significantly higher compared to the mean concentration of IL-6 (33.7±96.6 pg/mL) in the control group (p = 0.010). Moreover, the mean concentration of IL-18 (181.4±81.4 pg/mL) in ACS patients was significantly higher compared to the mean concentration (125.0±29.8 pg/mL) in the control group (p = 0.004), suggesting that both IL-6 and IL-18 were associated with ACS. However, there was no significant difference in the mean plasma levels of IL-1β (2.2±10.3 pg/mL) in the control group and ACS patients (0.1±5.5 pg/mL), and there was no significant difference in the mean plasma levels of IL-10 in the control group (1.6±5.2 ug/mL) and ACS patients (2.2±4.3 ug/mL) as shown in Table 3 and Figure 1.
Pearson’s correlation analysis revealed a positive correlation between IL-6 and IL-18 (R = 0.2290, p = 0.001) [Figure 2]. However, the correlation is not statistically significant between IL-10 and IL-18 (R = 0.0200, p = 0.360), and IL-6 and IL-1β (R = 0.0115, p = 0.490). Furthermore, a negative correlation exists between IL-18 and IL-1β (R = 0.0434, p = 0.180).
Table 1: Frequency (F) and percentage of participants (N = 43).
|
Gender
|
|
|
|
|
|
Male
|
10
|
50.0
|
17
|
73.9
|
|
Female
|
10
|
50.0
|
6
|
26.1
|
|
Age, years
|
|
|
|
|
|
15–24
|
6
|
30.0
|
0
|
0.0
|
|
25–64
|
14
|
70.0
|
19
|
82.6
|
Table 2: Clinical characteristics of the control group and acute coronary syndrome (ACS) patients.
|
Participants, n
|
20
|
23
|
|
Sex,n
|
|
Males
|
10
|
17
|
|
Females
|
10
|
6
|
|
Age, years
|
26.7 ± 6.0
|
52.7 ± 11.6
|
|
Weight, kg
|
60.3 ± 11.8
|
65.8 ± 8.2
|
|
Height, cm
|
163.0 ± 8.3
|
163.5 ± 7.1
|
|
Medical history, n (%)
|
|
|
|
Average BMI, kg/m2
|
22.5 ± 3.4
|
24.6 ± 3.3
|
|
Family history of CVD
|
0 (0.0)
|
5 (21.7)
|
|
Smoking
|
0 (0.0)
|
10 (43.5)
|
|
Hypertension
|
0 (0.0)
|
13 (56.5)
|
|
Drinking
|
0 (0.0)
|
0 (0.0)
|
|
Diagnostic history, mean ± SD
|
|
FBS
|
91.2 ± 15.4
|
139.6 ± 56.3
|
|
SBP, mm Hg
|
110.3 ± 10.0
|
134.4 ± 22.2
|
|
DBP, mm Hg
|
68.3 ± 7.5
|
87.0 ± 15.8
|
|
Total cholesterol, mmol/L
|
191.7 ± 38.7
|
174.0 ± 54.9
|
|
HDL, mmol/L
|
-
|
35.9 ± 8.4
|
|
LDL, mmol/L
|
-
|
109.3 ± 48.6
|
BMI: body mass index; CVD: cardiovascular disease; FBS: fasting blood glucose; SBP: systolic blood pressure; DBP: diastolic blood pressure; HDL: high-density lipoprotein; LDL: low-density lipoprotein; TG: total triglycerides.
Table 3: Plasma biomarker levels in each group.
|
IL-6
|
33.7 ± 96.6
|
19.40
|
21.6
|
298.6 ± 432.9
|
86.71
|
90.272
|
0.010
|
73.54
|
456.28
|
|
IL-18
|
125.0 ± 29.8
|
120.00
|
6.7
|
181.4 ± 81.4
|
165.10
|
16.98
|
0.004
|
18.99
|
93.77
|
|
IL-1β
|
2.2 ± 10.3
|
-1.34
|
2.3
|
0.1 ± 5.5
|
-1.36
|
1.15
|
0.410
|
-7.05
|
2.95
|
ACS: acute coronary syndrome; SEM: standard error of the median; LL: lower limit; UL: uper limit; IL: interleukin.
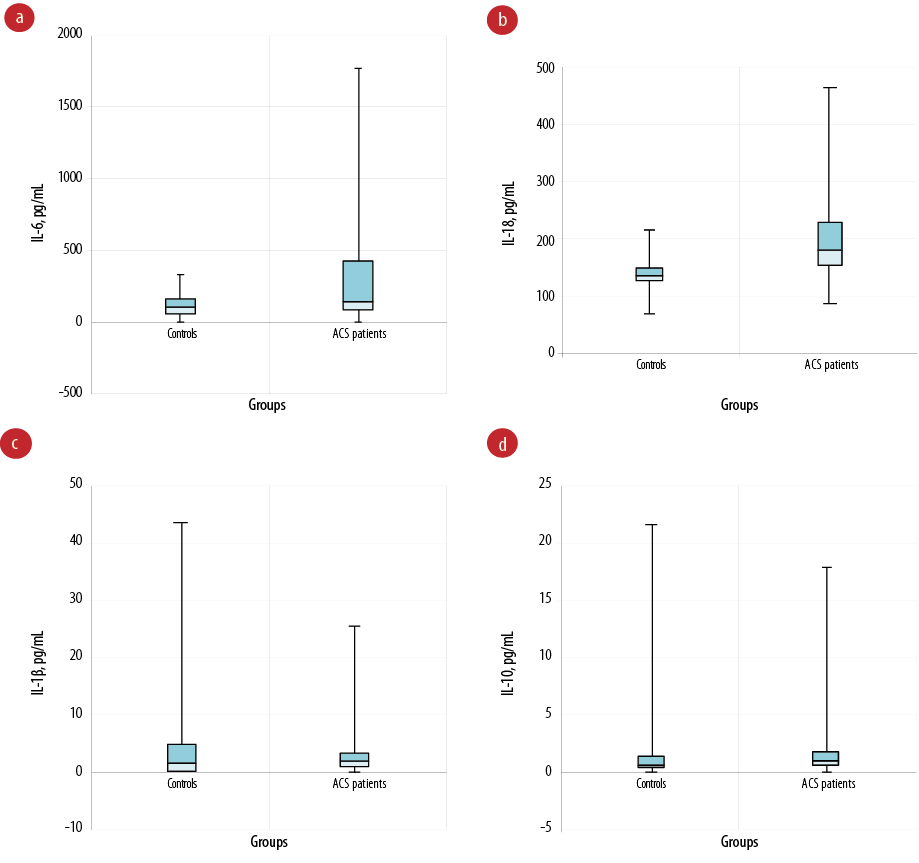 Figure 1: The plasma levels of interleukin (IL)-6, IL-18, IL-1β, and IL-10 in each group. (a) The plasma IL-6 levels in patients with acute coronary syndrome (ACS) were significantly higher than those of the control group; (b) the plasma IL-18 levels in patients with ACS were significantly higher than those of the control group; (c) no significant difference between plasma IL-1β levels in patients with ACS and control groups; (d) no significant difference between plasma IL-10 levels in patients with ACS and control group.
Figure 1: The plasma levels of interleukin (IL)-6, IL-18, IL-1β, and IL-10 in each group. (a) The plasma IL-6 levels in patients with acute coronary syndrome (ACS) were significantly higher than those of the control group; (b) the plasma IL-18 levels in patients with ACS were significantly higher than those of the control group; (c) no significant difference between plasma IL-1β levels in patients with ACS and control groups; (d) no significant difference between plasma IL-10 levels in patients with ACS and control group.
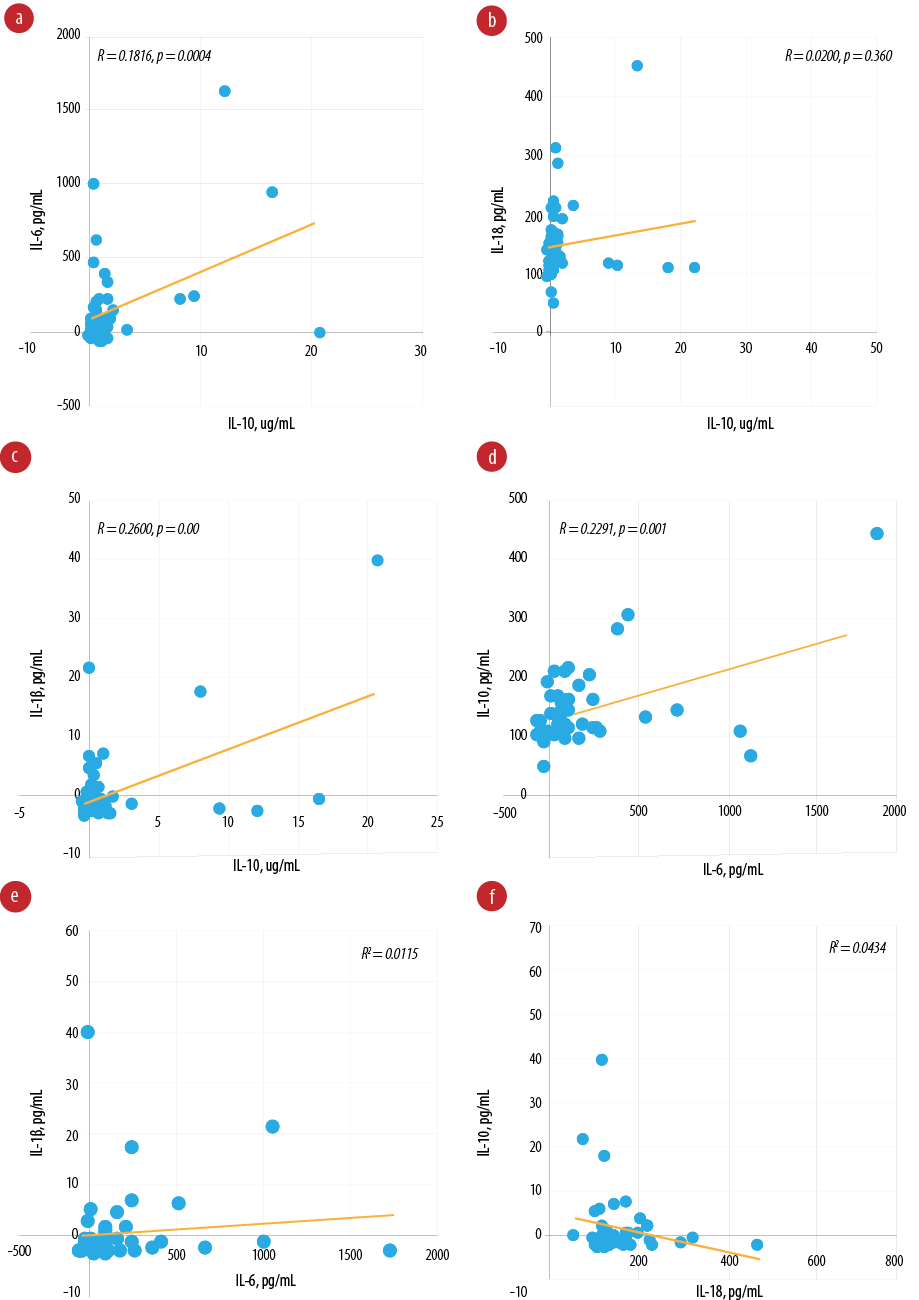 Figure 2: Pearson’s correlation between biomarkers. (a) Pearson’s correlation between interleukin (IL)-10 and IL-6 (R = 0.1816, p = 0.004); (b) Pearson’s correlation between IL-10 and IL-18 (R = 0.0200, p = 0.360); (c) Pearson’s correlation between IL-10 and IL-1β (R = 0.2600, p < 0.001); (d) Pearson’s correlation between IL-6 and IL-18 (R = 0.2291, p = 0.001); (e) Pearson’s correlation between IL-6 and IL-1β (R = 0.0115, p = 0.490); (f) Pearson's correlation between IL-18 and IL-1β (R = 0.0434, p = 0.180).
Figure 2: Pearson’s correlation between biomarkers. (a) Pearson’s correlation between interleukin (IL)-10 and IL-6 (R = 0.1816, p = 0.004); (b) Pearson’s correlation between IL-10 and IL-18 (R = 0.0200, p = 0.360); (c) Pearson’s correlation between IL-10 and IL-1β (R = 0.2600, p < 0.001); (d) Pearson’s correlation between IL-6 and IL-18 (R = 0.2291, p = 0.001); (e) Pearson’s correlation between IL-6 and IL-1β (R = 0.0115, p = 0.490); (f) Pearson's correlation between IL-18 and IL-1β (R = 0.0434, p = 0.180).
Discussion
Many research studies have proposed the clinical utilization of pro-inflammatory cardiac biomarkers. Higher IL-6, IL-18, and IL-1β levels and lower IL-10 levels are associated with ACS. The main findings of the present study were: (1) ACS patients showed higher IL-6 and IL-18 plasma levels compared to controls; (2) circulating levels of IL-1β and IL-10 showed no significant difference in both groups; and (3) IL-6 is positively correlated with IL-18 and IL-10 levels. IL-6 and IL-18 levels in plasma conform to previously conducted research. A previous study found that the concentration of IL-6 was significantly higher in ACS patients compared to healthy individuals,12 which is similar to our study. Moreover, an experimental study demonstrated that IL-18 enhances atherosclerosis by inducing expression of IL-6 in the vascular endothelial and smooth muscle cells,13 which is also endorsed by our study because a positive correlation exists between IL-6 and IL-18. Although research studies have revealed that pro-inflammatory cytokine IL-1β plays an important role in the pathogenesis of ACS and its inhibition has proven cardiovascular benefits, our study shows contrasting results that there is no significant difference between plasma IL-1β levels in ACS and control groups. On the contrary, a research study done by Parisi et al,14 showed that circulating levels of IL-1β were significantly higher in ACS patients compared to a control group. Another study revealed that IL-1β is known to drive the IL-6 signaling pathway,15 but our study showed a positive but non-significant correlation between IL-6 and IL-1β. However, the Canakinumab Anti-inflammatory Thrombosis Outcome Study proved the potential implication of IL-1β in conditioning the clinical course of patients after ACS.16 Admittedly, the technical difficulties in detecting IL-1β can result from the significant amount of pro-IL-l β that remains inside the cells and binds to large proteins.17 Therefore, the expected higher levels of IL-1β levels in ACS patients might have been masked. It is also possible that researchers are often less likely to report negative results for publication. Therefore, further research is required to validate the results. In ACS patients, low expression IL-10 in serum samples has been associated with an increased risk of cardiovascular events, and high IL-10 expression levels have been associated with a decreased risk.18,19 The level of IL-10 is lower in ACS patients compared to controls, but the difference is not statistically significant. This might be due to the small sample size. However, the elevated levels of IL-10 in ACS patients were positively correlated with IL-6 and IL-1β, which may be related to thrombosis, plaque rupture, and cardiac damage.
Our study is limited by its small sample size and there was a significant difference between the ages of the control group and ACS patients, which may have impacted the results. The strength of the study is that it is the first multimarker study that has evaluated IL-6, IL-18, IL-1β, and IL-10 levels together in ACS patients and all possible confounding factors were eliminated by careful consideration of the study design.
Conclusion
Levels of novel pro-inflammatory cytokines IL-6 and IL-18 were significantly increased in ACS patients, which supports the role of these biomarkers in ACS. Therefore, IL-6 and IL-18 can be considered potential targets for future therapeutic strategies using selective inhibitors for rationale and individualized therapy. However, a multimarker study that best assigns the cut-off values of these inflammatory cardiac biomarkers is needed for early diagnosis of ACS and to use them as therapeutic targets for individualized treatment of patients with ACS. A better understanding of the molecular biology of IL-6 and IL-18 should be considered to allow the successful development of their inhibitors. The clinical utility of these therapies should be considered a breakthrough in the management of ACS.
Disclosure
The authors declared no conflicts of interest. This research was funded by the World Class University grant ITB-2021 and Research ITB 2022.
Acknowledgments
The authors would like to acknowledge the staff of Hasan Sadikin Hospital for assisting in blood sample collection and the staff of the Pharmacology and Toxicology Laboratory of Bandung Institute of Technology for their assistance in performing the laboratory analysis.
references
- 1. Ji Q, Zeng Q, Huang Y, Shi Y, Lin Y, Lu Z, et al. Elevated plasma IL-37, IL-18, and IL-18BP concentrations in patients with acute coronary syndrome. Mediators Inflamm 2014;2014:165742.
- 2. Geovanini GR, Libby P. Atherosclerosis and inflammation: overview and updates. Clin Sci (Lond) 2018 Jun;132(12):1243-1252.
- 3. Ain QU, Sarfraz M, Prasesti GK, Dewi TI, Kurniati NF. Confounders in identification and analysis of inflammatory biomarkers in cardiovascular diseases. Biomolecules 2021 Oct;11(10):1464.
- 4. Fanola CL, Morrow DA, Cannon CP, Jarolim P, Lukas MA, Bode C, et al. Interleukin-6 and the risk of adverse outcomes in patients after an acute coronary syndrome: observations from the SOLID-TIMI 52 (stabilization of plaque using darapladib-thrombolysis in myocardial infarction 52) trial. J Am Heart Assoc 2017 Oct;6(10):e005637.
- 5. Anderson DR, Poterucha JT, Mikuls TR, Duryee MJ, Garvin RP, Klassen LW, et al. IL-6 and its receptors in coronary artery disease and acute myocardial infarction. Cytokine 2013 Jun;62(3):395-400.
- 6. Tøllefsen IM, Shetelig C, Seljeflot I, Eritsland J, Hoffmann P, Andersen GØ. High levels of interleukin-6 are associated with final infarct size and adverse clinical events in patients with STEMI. Open Heart 2021 Dec;8(2):e001869.
- 7. Kirii H, Niwa T, Yamada Y, Wada H, Saito K, Iwakura Y, et al. Lack of interleukin-1beta decreases the severity of atherosclerosis in ApoE-deficient mice. Arterioscler Thromb Vasc Biol 2003 Apr;23(4):656-660.
- 8. Viana-Huete V, Fuster JJ. Potential therapeutic value of interleukin 1b-targeted strategies in atherosclerotic cardiovascular disease. Rev Esp Cardiol (Engl Ed) 2019 Sep;72(9):760-766.
- 9. Li JJ, Guo YL, Yang YJ. Enhancing anti-inflammatory cytokine IL-10 may be beneficial for acute coronary syndrome. Med Hypotheses 2005;65(1):103-106.
- 10. Ammirati E, Cannistraci CV, Cristell NA, Vecchio V, Palini AG, Tornvall P, et al. Identification and predictive value of interleukin-6+ interleukin-10+ and interleukin-6- interleukin-10+ cytokine patterns in ST-elevation acute myocardial infarction. Circ Res 2012 Oct;111(10):1336-1348.
- 11. Fan S, He P, Guan J, Song W, Zhi H, Wang L. No association between interleukin-18 levels and risk of cardiovascular disease: a mendelian randomization study. Hereditas 2020 Apr;157(1):12.
- 12. Tang JN, Shen DL, Liu CL, Wang XF, Zhang L, Xuan XX, et al. Plasma levels of C1q/TNF-related protein 1 and interleukin 6 in patients with acute coronary syndrome or stable angina pectoris. Am J Med Sci 2015 Feb;349(2):130-136.
- 13. Gerdes N, Sukhova GK, Libby P, Reynolds RS, Young JL, Schönbeck U. Expression of interleukin (IL)-18 and functional IL-18 receptor on human vascular endothelial cells, smooth muscle cells, and macrophages: implications for atherogenesis. J Exp Med 2002 Jan;195(2):245-257.
- 14. Parisi V, Petraglia L, Cabaro S, D’Esposito V, Bruzzese D, Ferraro G, et al. Imbalance between interleukin-1β and interleukin-1 receptor antagonist in epicardial adipose tissue is associated with non ST-segment elevation acute coronary syndrome. Front Physiol 2020 Feb;11:42.
- 15. Ridker PM. From C-reactive protein to interleukin-6 to interleukin-1: moving upstream to identify novel targets for atheroprotection. Circ Res 2016 Jan;118(1):145-156.
- 16. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al; CANTOS Trial Group. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017 Sep;377(12):1119-1131.
- 17. Borth W, Urbanski A, Prohaska R, Susanj M, Luger TA. Binding of recombinant interleukin-1 beta to the third complement component and alpha 2-macroglobulin after activation of serum by immune complexes. Blood 1990 Jun;75(12):2388-2395.
- 18. Al Shahi H, Shimada K, Miyauchi K, Yoshihara T, Sai E, Shiozawa T, et al. Elevated circulating levels of inflammatory markers in patients with acute coronary syndrome. Int J Vasc Med 2015;2015:805375.
- 19. Mizia-Stec K, Gasior Z, Zahorska-Markiewicz B, Janowska J, Szulc A, Jastrzebska-Maj E, et al. Serum tumour necrosis factor-alpha, interleukin-2 and interleukin-10 activation in stable angina and acute coronary syndromes. Coron Artery Dis 2003 Sep;14(6):431-438.