Uterine fibroids, also known as leiomyomas/myomas/fibroids, are monoclonal tumors of smooth muscle cells of the uterus made up of a substantial amount of extracellular matrix containing proteoglycan, fibronectin, and collagen. They can be single or multiple and are characterized by their location in relation to the layers of the uterus (intramural, subserosal, or submucosal).1,2 They are the most common benign uterine neoplasms that develop in women in their reproductive ages.3 Despite their unclear pathophysiology, the literature suggests that estrogens and progesterone promote the growth of this tumor, as fibroids rarely appear before menarche and regress after menopause.4 Black women, obese women, nulliparous women, and women with a positive family history of fibroids are more likely to develop them.5,6
Most research about fibroids focused on women seeking medical care, thus the actual incidence of uterine fibroids in the general population is unknown.7,8 The prevalence of fibroids has been estimated at between 3.3% and 77%, which varies with age and ethnicity.8,9 In a study conducted in the USA, the incidence of uterine fibroids among Black American women was 60% by age 35, increasing to above 80% by age 50, whereas the incidence among Caucasian women was 40% by age 35, and nearly 70% by age 50.10 In Europe, a similar trend of higher incidence of fibroids among women of African origin has also been reported. Black women also report significantly more severe fibroid symptoms.11 Given this severity of the risk of fibroids in black women, there is a significant under-representation of African women in the available literature, resulting in the underestimation of the occurrence of uterine fibroids among women of African descent.9,12
The majority of women with uterine fibroids are asymptomatic, and consequently get less clinical attention as fibroid tumors often remain undiagnosed. Although bleeding and pelvic pain are the main symptoms frequently reported in the literature in relation to uterine fibroids, the number of systematic studies on the symptomatology of fibroids is limited.13,14 However, women with uterine fibroids may suffer more often from dyspareunia and non-cyclic pelvic pain. Large fibroids that result in the enlargement of the uterus have been linked with bowel and bladder problems as well as abdominal protrusion.15 Fibroids can cause painful menses, infertility, and recurrent miscarriages. Heavy menstrual bleeding in women with symptomatic fibroids stops when they reach menopause, at this stage, symptoms improvement is also experienced with fibroid shrinkage.15–17
During ultrasonography (US) examination, fibroids typically are seen as well-circumscribed round concentric solid myometrial mass or attached to the myometrium, which show a variable amount of acoustic shadowing at the edge of the lesion and/or fan-shaped shadowing. These may show a variable echogenicity that presents as hypoechoic, hyperechoic, or isoechoic lesions and in some cases, areas of calcifications, and cystic changes. These may demonstrate peripheral blood flow on color and power Doppler. The most common differential diagnosis for uterine fibroids that may be misdiagnosed is adenomyosis, which sonographically presents as globular uterine enlargement without the presence of well-defined leiomyomata, and is typically ill-defined lesions within the myometrium in one or more sites in the uterine wall or affecting most of the myometrium. It may show intralesional flow on color or power Doppler. This sonographic differentiation between leiomyomas and adenomyosis is based on the Morphological Uterus Sonographic Assessment (MUSA) criteria.18
Uterine myomas can be treated in a variety of ways, from medication to surgery. Treatment is frequently reserved for symptomatic myomas.19 US is the first line imaging for the evaluation of myomas, whether clinically suspected or not because it is inexpensive and available in many healthcare facilities in low-resourced settings such as Africa, even though the more expensive magnetic resonance imaging is the gold standard.20,21
There are some studies published internationally on the sonographic features of uterine fibroids with varying outcomes, however; very few studies exist or have been conducted in Africa, specifically Ghana.21–23 As a result, our study was conducted to determine the clinical presentations and ultrasonographic features of uterine fibroids in adult Ghanaians. We also sought to determine the possible association between the clinical presentation/indication, sonographic features of uterine fibroids, and age distribution. This is an important study since it offers another layer of knowledge to the management of uterine fibroids.
Methods
The US reports of all patients who had US examinations at the Cape Coast Teaching Hospital (CCTH) between 1 January 2018 and 31 December 2021 were retrieved and evaluated in this retrospective study for only cases diagnosed with uterine fibroids. As the largest public health center in the Central Region of Ghana, CCTH is one of Ghana’s leading research institutions, as well as a training center for residents in a variety of medical specialties. It also serves as a clinical teaching center for the University of Cape Coast Medical School.
We obtained all the records of ultrasound-diagnosed cases of uterine fibroids from the Lightwave Health Information Management System, which contains all detailed electronic health records of patients using keywords like; fibroids, myomas, uterine fibroids, uterine myomas, and leiomyomas. The unique identification number for each patient with uterine fibroids was retrieved to access the patient’s US reports and the clinical presentations/indications, and also to avoid duplication of cases. This was done for all the patients diagnosed with fibroids over the study period. From the reports obtained, the ages and imaging features of the patients were retrieved. The sonographic imaging features were then categorized under these themes; fibroid homogeneity/heterogeneity, fibroid echopattern, fibroid outline and whether there were degenerative changes. We also obtained as to whether the fibroid nodules were solitary (one nodule) or multiple (two or more nodules). A total of 4279 patients with uterine fibroids was consecutively retrieved for analyses.
The US images were acquired through a transabdominal route on a full urinary bladder or transvaginal route. Mindray Diagnostic Digital Ultrasound System, Model DCN3, with a 3.5 MHz convex probe or 7.5 MHz transvaginal probe made by Shenzhen Mindray Bio-Medical Electronics Company Limited (Nanshan, Shenzhen, China) was used for the US examinations. All the US scanning and reports were done by three radiologists who had already completed the US imaging examination and reporting learning curve; each with over 10 years of gynecological ultrasonographic practice.
With the help of LibreOffice Calc version 1:6.1.5-3+deb10u6 developed by the Document Foundation and SPSS (IBM Corp. Released 2013. IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp.), the data obtained (age, clinical presentations, and ultrasonographic features) were analyzed and presented in appropriate tables and charts after rigorous scrutiny, which included organization, arrangement, and coding. We analyzed the distribution of cases across age groups using the World Health Organization age categorization of ‘15–19 years’, ‘20–24 years’, ‘25–29 years’, ‘30–34 years’, ‘35–39 years’, ‘40–44 years’, ‘45–49 years’, ‘50–54 years’, ‘55–59 years’, and ‘≥ 60 years’.24 We further reclassified these groups into ‘15–29 years’, ‘30–44 years’, ‘45–59 years’, and ‘≥ 60 years’ and used a chi-squared test to check for possible associations between the clinical presentations/indications, age distribution, and ultrasonographic features of uterine fibroids. All the estimations for this study were done with a p-value ≤ 0.05 as the level of statistical significance.
The Ethical Review Committee of CCTH provided the study with an ethical clearance number CCTHERC/EC/2020/094. As this was a retrospective study, informed consent was not required but anonymity and confidentiality were guaranteed. The study complied with the 1975 Helsinki Declaration.
Results
Over the course of the four-year study period, 4279 women between the ages of 16 and 69 were diagnosed with uterine fibroids via US. The mean age was 37.1±11.5 years. Multiple fibroids accounted for the majority of the fibroid cases recorded (n = 2633, 61.5%) [Table 1].
Table 1: Characteristics of patients and the number of fibroid nodules.
|
Total count
|
4279
|
100
|
|
Age, years
|
|
|
|
Minimum
|
16
|
-
|
|
Maximum
|
69
|
-
|
|
Mean ± SD
|
37.1 ± 11.5
|
-
|
|
Number of fibroid nodules
|
|
Solitary nodules
|
1646
|
38.5
|
Routine checkup (n = 1310, 28.1%) was the leading asymptomatic indication for women who were diagnosed with uterine fibroids in this study. The second and third indications, respectively, were menorrhagia (n = 1104, 23.7%) and lower abdominal mass (n = 801, 17.2%). Figure 1 shows a detailed overview of the indications for women diagnosed with uterine fibroids via sonographic evaluation in this current study.
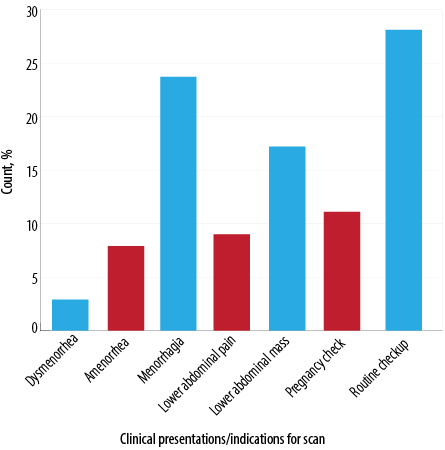 Figure 1: Indications for patients diagnosed
Figure 1: Indications for patients diagnosed
with fibroids.
Findings from Table 2 showed that the majority of the patients were symptomatic (n = 2451, 57.3%). Menorrhagia (n = 1104, 39.1%) and lower abdominal mass (n = 801, 28.3%) were the topmost symptomatic indications.
Table 2: Distribution of the clinical presentations of uterine fibroid patients by symptomatic and asymptomatic presentations.
|
Symptomatic*
|
2451
|
57.3
|
|
Dysmenorrhea
|
137
|
4.8
|
|
Amenorrhea
|
366
|
12.9
|
|
Menorrhagia
|
1104
|
39.1
|
|
Lower abdominal pain
|
419
|
14.8
|
|
Lower abdominal mass
|
801
|
28.3
|
|
Asymptomatic
|
1828
|
42.7
|
|
Routine checkup
|
1310
|
71.7
|
*The total percentages generated from the SPSS do not add up to 100.0% due to figure rounding.
Almost all (n = 4125, 96.4%) of the fibroid nodules sonographically exhibited a smooth regular outline and were primarily heterogeneous nodules (n = 3282, 76.7%) [Figure 2]. In relation to the fibroid echopattern, most of the nodules were hypoechoic (n = 3358, 51.1%) [Figure 2], followed by hyperechoic nodules (n = 2554, 38.9%) [Figure 3]. The number of degenerative changes accounted for less than a third of all uterine fibroids nodules, with the commonest degenerative feature being solid nodules with rim of calcification and areas of calcified degeneration (n = 965, 94.6%) [Figure 4] and nodules with cystic areas being the lesser observed degenerative feature (n = 55, 5.4%) [Figure 5]. Detailed sonographic findings can be found in Table 3.
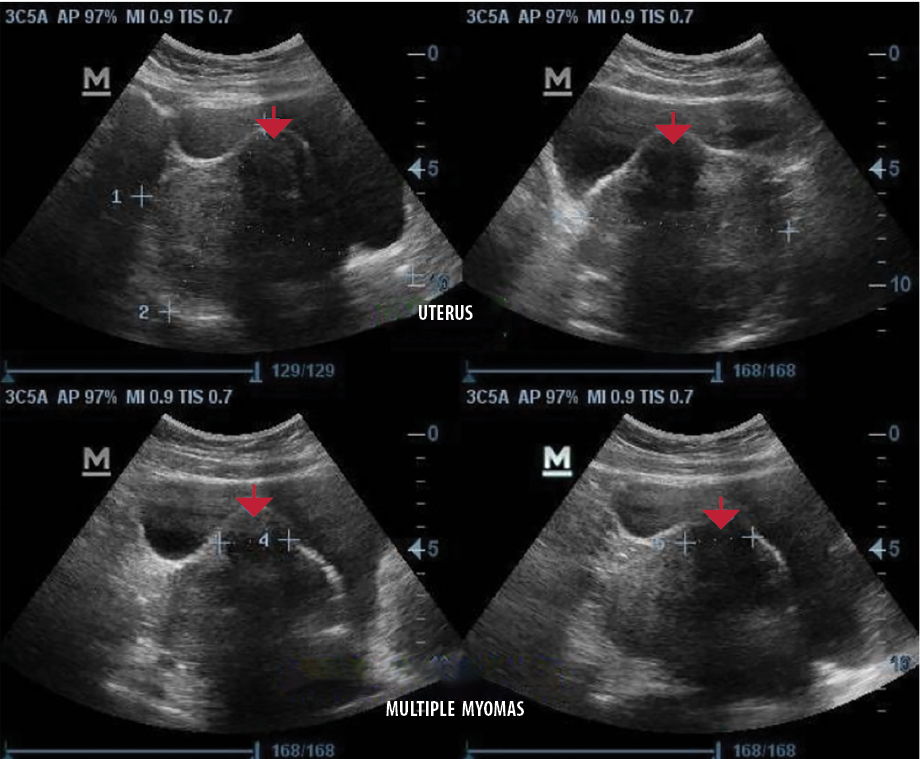 Figure 2: Transabdominal sonograms of the uterus showing a heterogeneous but predominantly hypoechoic fibroid nodule with a smooth regular outline at the anterior part (red arrows).
Figure 2: Transabdominal sonograms of the uterus showing a heterogeneous but predominantly hypoechoic fibroid nodule with a smooth regular outline at the anterior part (red arrows).
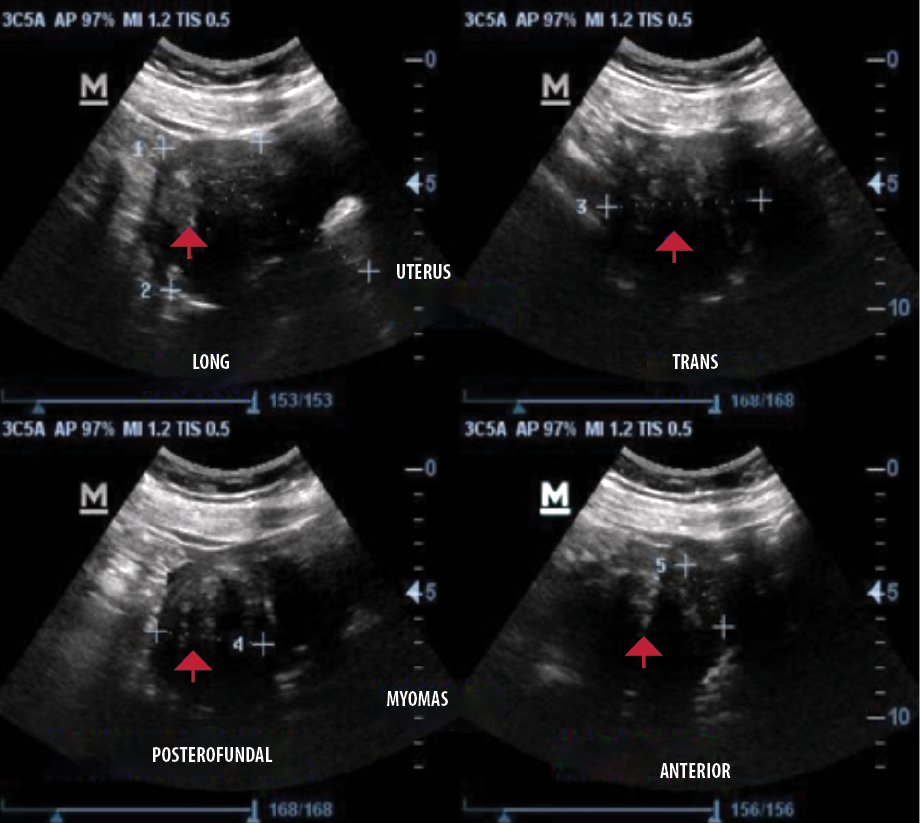 Figure 3: Transabdominal sonograms of the uterus showing a heterogeneous but predominantly hyperechoic fibroid nodule at the posterior part (red arrows).
Figure 3: Transabdominal sonograms of the uterus showing a heterogeneous but predominantly hyperechoic fibroid nodule at the posterior part (red arrows).
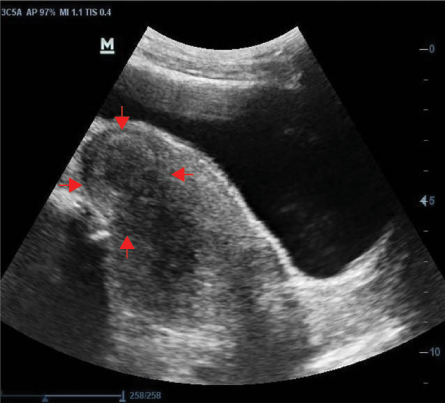 Figure 4: Transabdominal sonogram of the uterus showing a heterogeneous fibroid nodule with a rim of calcific degeneration (red arrows).
Figure 4: Transabdominal sonogram of the uterus showing a heterogeneous fibroid nodule with a rim of calcific degeneration (red arrows).
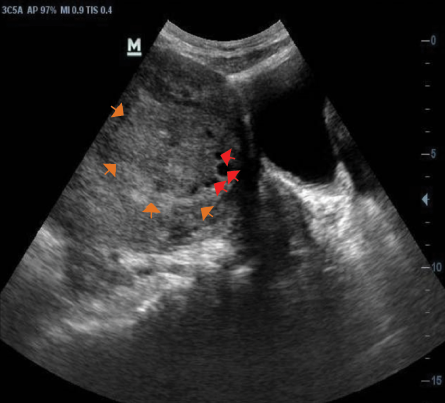 Figure 5: Transabdominal sonogram of the uterus showing an isoechoic fibroid nodule (orange arrows) with areas of cystic degeneration (red arrows).
Figure 5: Transabdominal sonogram of the uterus showing an isoechoic fibroid nodule (orange arrows) with areas of cystic degeneration (red arrows).
Table 3: Sonographic features of patients diagnosed with uterine fibroids.
|
Fibroid homogeneity/heterogeneity
|
|
Heterogeneous nodule
|
3282
|
76.7
|
|
Homogenous nodule
|
997
|
23.3
|
|
Fibroid echo pattern*
|
|
|
|
Hypoechoic nodule
|
3358
|
51.1
|
|
Hyperechoic nodule
|
2554
|
38.9
|
|
Isoechoic nodule
|
661
|
10.1
|
|
Fibroid outline
|
|
|
|
Regular smooth fibroid nodule
|
4125
|
96.4
|
|
Irregular fibroid nodule
|
154
|
3.6
|
|
Degenerative changes
|
|
|
|
Solid with rim of calcification and areas of degeneration
|
965
|
94.6
|
*The total Percentages generated from the SPSS do not add up to 100.0% due to figure rounding.
As shown in Figure 6, the prevalence of fibroids rises initially, peaks at 30–34 years, and then declines, with the lowest occurring in the ≥ 60 group.
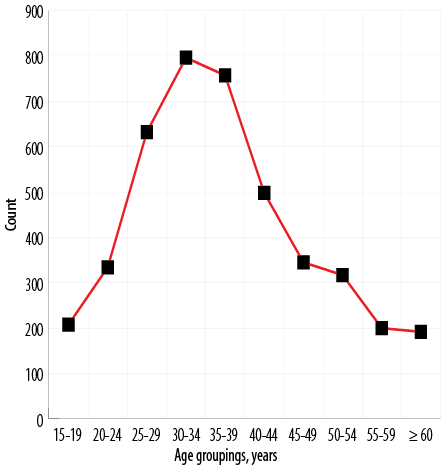 Figure 6: Distribution of uterine fibroids in various age groups.
Figure 6: Distribution of uterine fibroids in various age groups.
Comparative analysis of the sonographic features and the age distribution of the patients revealed significant associations between fibroid homogeneity/heterogeneity (p ≤ 0.001), outline (p ≤ 0.001) and the age groupings. This implies a significant number of fibroid cases under these themes were seen in the 30–44 years age group. A similar pattern was observed under the fibroid echo pattern (hypoechoic, hyperechoic, and isoechoic) where most of the cases were also significantly observed in the 30–44 years age group (p ≤ 0.001). The degenerative changes for the fibroid nodules also exhibited a similar pattern of association (p ≤ 0.001). Detailed findings can be found in Table 4.
Table 4: Relationship between the sonographic features and the age distribution of fibroid-diagnosed patients.
|
Fibroid homogeneity/heterogeneity
|
|
Heterogeneous nodule
|
762 (23.2)
|
1580 (48.1)
|
748 (22.8)
|
192 (5.9)
|
≤ 0.001
|
|
Homogenous nodule
|
412 (41.3)
|
471 (47.2)
|
114 (11.4)
|
0 (0.0)
|
|
|
Fibroid echo pattern
|
|
Hypoechoic nodule
|
|
|
|
|
|
|
Yes
|
797 (23.7)
|
1601 (47.7)
|
768 (22.9)
|
192 (5.7)
|
≤ 0.001
|
|
No
|
377 (40.9)
|
450 (48.9)
|
94 (10.2)
|
0 (0.0)
|
|
|
Hyperechoic nodule
|
|
Yes
|
563 (22.0)
|
1178 (46.1)
|
623 (24.4)
|
190 (7.4)
|
≤ 0.001
|
|
No
|
611 (35.4)
|
873 (50.6)
|
239 (13.9)
|
2 (0.1)
|
|
|
Isoechoic nodule
|
|
Yes
|
292 (44.2)
|
303 (45.8)
|
66 (10.0)
|
0 (0.0)
|
≤ 0.001
|
|
No
|
882 (24.4)
|
1748 (48.3)
|
796 (22.0)
|
192 (5.3)
|
|
|
Fibroid outline
|
|
Regular smooth fibroid Nodule
|
1147 (27.8)
|
1955 (47.4)
|
831 (20.1)
|
192 (4.7)
|
≤ 0.001
|
|
Irregular fibroid nodule
|
27 (17.5)
|
96 (62.3)
|
31 (20.1)
|
0 (0.0)
|
|
|
Degenerative changes
|
|
|
|
|
|
|
Solid with rim of calcification and areas of degeneration
|
|
Yes
|
148 (15.3)
|
449 (46.5)
|
272 (28.2)
|
96 (9.9)
|
≤ 0.001
|
|
No
|
1026 (31.0)
|
1602 (48.3)
|
590 (17.8)
|
96 (2.9)
|
|
|
Solid with cystic areas
|
|
|
|
|
|
|
Yes
|
0 (0.0)
|
20 (36.4)
|
35 (63.6)
|
0 (0.0)
|
≤ 0.001
|
Table 5: Distribution showing the associations between the presenting indications and the age distribution of fibroid-diagnosed patients.
|
Dysmenorrhea
|
|
|
|
|
|
|
Yes
|
81 (59.1)
|
56 (40.9)
|
0 (0.0)
|
0 (0.0)
|
≤ 0.001
|
|
No
|
1093 (26.4)
|
1995 (48.2)
|
862 (20.8)
|
192 (4.6)
|
|
|
Amenorrhea
|
|
|
|
|
|
|
Yes
|
254 (69.4)
|
96 (26.2)
|
16 (4.4)
|
0 (0.0)
|
≤ 0.001
|
|
No
|
920 (23.5)
|
1955 (50.0)
|
846 (21.6)
|
192 (4.9)
|
|
|
Menorrhagia
|
|
|
|
|
|
|
Yes
|
299 (27.1)
|
701 (63.5)
|
104 (9.4)
|
0 (0.0)
|
≤ 0.001
|
|
No
|
875 (27.6)
|
1350 (42.5)
|
758 (23.9)
|
192 (6.0)
|
|
|
Lower abdominal pain
|
|
|
|
|
|
|
Yes
|
17 (4.1)
|
40 (9.5)
|
262 (62.5)
|
100 (23.9)
|
≤ 0.001
|
|
No
|
1157 (30.0)
|
2011 (52.1)
|
600 (15.5)
|
92 (2.4)
|
|
|
Lower abdominal mass
|
|
|
|
|
|
|
Yes
|
14 (1.7)
|
136 (17.0)
|
459 (57.3)
|
192 (24.0)
|
≤ 0.001
|
|
No
|
1160 (33.4)
|
1915 (55.1)
|
403 (11.6)
|
0 (0.0)
|
|
|
Pregnancy check
|
|
|
|
|
|
|
Yes
|
228 (44.0)
|
278 (53.7)
|
12 (2.3)
|
0 (0.0)
|
≤ 0.001
|
|
No
|
946 (25.2)
|
1773 (47.1)
|
850 (22.6)
|
192 (5.1)
|
|
|
Routine checkup
|
|
|
|
|
|
|
Yes
|
299 (22.8)
|
817 (62.4)
|
194 (14.8)
|
0 (0.0)
|
≤ 0.001
|
Table 6: Comparison of the sonographic features of fibroids and amenorrhea in pregnant women versus non-pregnant women.
|
Fibroid homogeneity/heterogeneity
|
|
|
|
Heterogeneous nodule
|
479 (59.1)
|
2803 (80.8)
|
|
Homogenous nodule
|
331 (40.9)
|
666 (19.2)
|
|
Fibroid echo pattern
|
|
|
|
Hypoechoic nodule
|
543 (47.0)
|
2815 (52.0)
|
|
Hyperechoic nodule
|
408 (35.3)
|
2146 (39.6)
|
|
Isoechoic nodule
|
204 (17.7)
|
457 (8.4)
|
|
Fibroid outline
|
|
|
|
Regular smooth fibroid nodule
|
781 (96.4)
|
3344 (96.4)
|
|
Irregular fibroid nodule
|
29 (3.6)
|
125 (3.6)
|
|
Degenerative changes
|
|
|
|
Solid with rim of calcification and areas of degeneration
|
192 (98.0)
|
773 (93.8)
|
|
Solid with cystic areas
|
4 (2.0)
|
51 (6.2)
|
The younger patients in this current study significantly presented with dysmenorrhea, amenorrhea, menorrhagia, and for routine checkup (p ≤ 0.001) whilst lower abdominal pain and lower abdominal mass were significantly more common clinical presentations in the older age categories (p ≤ 0.001) [Table 5].
Out of the total number of women diagnosed with fibroids, 810 (18.9%) were pregnant. Many of the sonographic features were more common in the non-gravid women except for homogenous nodule (40.9% vs. 19.2%), isoechoic nodule (17.7% vs. 8.4%), irregular fibroid nodule (3.6% vs. 0.7%), and nodules with rim of calcification and areas of calcified degeneration (98.0% vs. 93.8%) as shown in Table 6. Out of the patients who presented with amenorrhea, 62.6% (n = 229) were pregnant and 37.4% (n = 137) were not.
Discussion
Despite literature on the high risk of developing fibroids in women of African descent, very few studies have been conducted in these settings to clearly illustrate the reality on the ground.25 Morbidity as a result of symptomatic fibroids has an accompanying high healthcare cost and a lower quality of life. In the USA, for example, there is a considerable degree of morbidity in premenopausal women diagnosed with fibroids predicted to cost approximately $5.9 to $34.4 billion annually in societal costs.26 With race as a major risk factor, the financial burden of this neoplasm may be greater on African countries.
In this current investigation, the majority of the fibroid nodules were multiple (61.5%). This corroborates the findings of studies from the UK and Pakistan, which reported multiple fibroids occurrence of 60.7% and 63.1%, respectively.27,28 Contrary to this, a study from Slovenia reported a higher number of solitary fibroid nodules (57.9%).29 This study showed that fibroid nodules were more common in women of reproductive age (15–49 years), accounting for 83.4% of all cases. This is consistent with what has already been described in the literature.25,30
The average age of women diagnosed with fibroids was 37.1±11.5 years. This is comparable to the average ages of 35.8±7.6 years and 35.7±6.1 years reported in Kano and Anambra States in Nigeria, respectively.31,32 An international internet-based survey of 21 746 women mostly from the USA and Europe found a higher mean age of 40.4±6.9 years.33 Another study in the USA found an average age of 46.5±6.6 years.34 This demonstrates that Africans as a whole may be developing fibroids at an earlier age than those in the western world. This could be attributable in part to changes in environmental and biological factors such as diet and skin color among others. Darker skin has been reported to hinder the formation of biologically active vitamin D, which has been linked to an increased risk of fibroid tumors.35 However, another study in Oman reported a comparatively lower mean age of 32.6±4.2 years. 36 The reason for this finding was not readily elucidated.36 Furthermore, studies have reported that women of African descent diagnosed with fibroids experience the most severe symptoms and are thus more likely to be diagnosed earlier due to early presentations to a health facility.2,35 This is supported by findings in our study which demonstrates that the majority (57.3%) of the patients were experiencing symptoms.
Despite the fact that uterine fibroids can cause a variety of symptoms, there are a few of these symptoms that are particularly common. However, due to their variability in different patients, these symptoms are poor markers when trying to figure out a diagnosis.7,37 Nonetheless, it is vital to keep track of them, as this will allow for comparisons in different jurisdictions for better understanding. The leading indication in this present study for women with asymptomatic uterine fibroids was routine checkup. However, for symptomatic fibroids, we found menorrhagia and lower abdominal mass as the top two most common. This trend has been corroborated by a study in Nigeria.31 Another study from the USA also reported abnormal uterine bleeding, abdominal bloating, and pelvic discomfort due to mass effect as their most common symptoms.38 This depicts the diverse presentations in relation to uterine fibroids. We realized that some clinical presentations (dysmenorrhea, amenorrhea, menorrhagia, and routine checkup) were significantly more common in the younger age groups (p ≤ 0.001), whereas lower abdominal pain and lower abdominal mass were more common in the older age
groups (p ≤ 0.001).
US provides a platform that ensures the distinction between possible differentials allowing for the clear diagnosis of uterine fibroids, thereby helping in the institution of the correct therapeutic interventions. Also, owing to its low access cost, it has become a modality which can clearly provide the actual situation in relation to uterine fibroids in poorly resourced settings.39 Findings from this study compliant with the MUSA criteria, showed that uterine fibroids were mostly hypoechoic heterogeneous nodules with a smooth regular outline similar to what was reported by Khan et al,13 who sonographically described fibroids as well-defined, solid masses with a whorled appearance. They further posited that the nodules were usually isoechoic, but sometimes may be hypoechoic. This has been corroborated by Shwayder et al.40 According to McLucas et al,41 arterial supply to myomas is less than that of the normal myometrium, and a myoma may degenerate when it outgrows its blood supply. In our study, the number of degenerative changes accounted for less than a third of all uterine fibroid nodules. Almost all the sonographic features in this study were significantly seen in the 30–44 years age group (p ≤ 0.001). McLucas et al,41 reported further that, the end stage of fibroid degeneration is calcification and usually occurs after menopause. It can be found in up to 10% of myomas and can be easily identified on computed tomography or US.40,41 We however found a higher prevalence (965/4279, 22.6%) of calcific degeneration with a significantly higher occurrence in the relatively older women. Similar reasons as reported by McLucas et al,41 above can be ascribed to this assertion. Also, homogenous nodules, isoechoic nodules, irregular fibroid nodules, and nodules with the rim of calcification and areas of calcified degeneration were comparatively more common in gravid women in the current study. Reasons for this observation could not be elucidated from this study and from the reviewed literature.
Fibroids are a major complication in obstetric practice observed at a rate of 10–40%, but its prevalence in the USA is estimated between 0.1% and 10.7%.42 In this current study, however, we found a higher percentage of our patients (18.9%) to be pregnant. The reasons for the higher prevalence of uterine fibroids in our pregnant population could be attributed to the earlier stated reasons of differences in environmental and biological factors coupled with the higher fertility rate of > 4.01 births per woman in Africa as a whole, and 3.8 births per woman in Ghana to be specific compared to the average of < 1.82 births per woman in the western world.35,43
Our study has some limitations. Uterine fibroid patients whose sonographic findings were not found in our system were excluded. The locations of uterine fibroids were not included in the study, which may be another limitation. Histopathology was not considered for the fibroid nodules as this study focused mainly on the sonographic features which is another possible limitation.
Conclusion
Uterine fibroids were sonographically hypoechoic heterogeneous nodules with a smooth regular outline with a significant occurrence in the 30–44 years age group. The most common symptomatic clinical presentations of fibroids were menorrhagia and lower abdominal mass. Easier diagnosis of uterine fibroid vis a vis its management, involves the recognition of the varied imaging appearances and clinical presentations, especially in women experiencing symptoms, as this will enhance the quality of life for such women, with enormous societal benefits.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
Acknowledgments
We are grateful to the management of the Radiology Department and the staff of the ultrasound unit of this department for their support.
references
- 1. Stewart EA. Clinical practice. Uterine fibroids. N Engl J Med 2015 Apr;372(17):1646-1655.
- 2. Marsh EE, Al-Hendy A, Kappus D, Galitsky A, Stewart EA, Kerolous M. Burden, prevalence, and treatment of uterine fibroids: a survey of US women. J Womens Health (Larchmt) 2018 Nov;27(11):1359-1367.
- 3. Okolo S. Incidence, aetiology and epidemiology of uterine fibroids. Best Pract Res Clin Obstet Gynaecol 2008 Aug;22(4):571-588.
- 4. Donnez J. Uterine fibroids and progestogen treatment: lack of evidence of its efficacy: a review. J Clin Med 2020 Dec;9(12):3948.
- 5. De La Cruz MS, Buchanan EM. Uterine fibroids: diagnosis and treatment. Am Fam Physician 2017 Jan;95(2):100-107.
- 6. Klatsky PC, Tran ND, Caughey AB, Fujimoto VY. Fibroids and reproductive outcomes: a systematic literature review from conception to delivery. Am J Obstet Gynecol 2008 Apr;198(4):357-366.
- 7. Al-Hendy A, Myers ER, Stewart E. Uterine fibroids: burden and unmet medical need. Semin Reprod Med 2017 Nov;35(6):473-480.
- 8. Ghosh S, Naftalin J, Imrie R, Hoo WL. Natural history of uterine fibroids: a radiological perspective. Curr Obstet Gynecol Rep 2018;7(3):117-121.
- 9. Stewart EA, Cookson CL, Gandolfo RA, Schulze-Rath R. Epidemiology of uterine fibroids: a systematic review. BJOG 2017 Sep;124(10):1501-1512.
- 10. Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol 2003 Jan;188(1):100-107.
- 11. Giri A, Edwards TL, Hartmann KE, Torstenson ES, Wellons M, Schreiner PJ, et al. African genetic ancestry interacts with body mass index to modify risk for uterine fibroids. PLoS Genet 2017 Jul;13(7):e1006871.
- 12. Elugwaraonu O, Okojie AI, Okhia O, Oyadoghan GP. The incidence of uterine fibroid among reproductive age women: a five year review of cases at ISTH, Irrua, Edo, Nigeria. J. Basic Appl Innov Res 2013;2(3):55-60.
- 13. Khan AT, Shehmar M, Gupta JK. Uterine fibroids: current perspectives. Int J Womens Health 2014 Jan;6:95-114.
- 14. Pritts EA, Olive DL. When should uterine fibroids be treated? Curr Obstet Gynecol Rep 2012 Jun;1(2):71-80.
- 15. Doherty L, Mutlu L, Sinclair D, Taylor H. Uterine fibroids: clinical manifestations and contemporary management. Reprod Sci 2014 Sep;21(9):1067-1092.
- 16. Donnez J, Dolmans MM. Uterine fibroid management: from the present to the future. Hum Reprod Update 2016 Nov;22(6):665-686.
- 17. Tapmeier TT, Uimari O, Nazri HM. Endometriosis and uterine fibroids (leiomyomata): comorbidity, risks and implications. Front. Reprod Health 2021 Oct; 3:750018.
- 18. Van den Bosch T, Dueholm M, Leone FP, Valentin L, Rasmussen CK, Votino A, et al. Terms and definitions for describing myometrial pathology using ultrasonography. Ultrasound Obstet Gynecol 2015;46(3):284-298.
- 19. Al-Shukri M, Al-Ghafri W, Al-Dhuhli H, Gowri V. Vaginal myomectomy for prolapsed submucous fibroid: it is not only about size. Oman Med J 2019 Nov;34(6):556-559.
- 20. Edzie EK, Dzefi-Tettey K, Gorleku PN, Amankwa AT, Aidoo E, Agyen-Mensah K, et al. Evaluation of the clinical and imaging findings of breast examinations in a tertiary facility in Ghana. Int J Breast Cancer 2021 Jul;2021:5541230.
- 21. Testa AC, Di Legge A, Bonatti M, Manfredi R, Scambia G. Imaging techniques for evaluation of uterine myomas. Best Pract Res Clin Obstet Gynaecol 2016 Jul;34:37-53.
- 22. Woźniak A, Woźniak S. Ultrasonography of uterine leiomyomas. Prz Menopauzalny 2017 Dec;16(4):113-117.
- 23. Wilde S, Scott-Barrett S. Radiological appearances of uterine fibroids. Indian J Radiol Imaging 2009 Jul-Sep;19(3):222-231.
- 24. Ahmad OB, Boschi-Pinto C, Lopez AD, Murray CJ, Lozano R, Inoue M. Age standardization of rates: a new WHO standard. Geneva: World Health Organization. 2001 Jan;9(10):1-4.
- 25. Wise LA, Laughlin-Tommaso SK. Epidemiology of uterine fibroids–from menarche to menopause. Clin Obstet Gynecol 2016 Mar;59(1):2-24.
- 26. Styer AK, Rueda BR. The epidemiology and genetics of uterine leiomyoma. Best Pract Res Clin Obstet Gynaecol 2016 Jul;34:3-12.
- 27. Mavrelos D, Ben-Nagi J, Holland T, Hoo W, Naftalin J, Jurkovic D. The natural history of fibroids. Ultrasound Obstet Gynecol 2010 Feb;35(2):238-242.
- 28. Begum S, Khan S. Audit of leiomyoma uterus at Khyber teaching hospital Peshawar. J Ayub Med Coll Abbottabad 2004 Apr-Jun;16(2):46-49.
- 29. Bizjak T, Bečić A, But I. Prevalence and risk factors of uterine fibroids in North-East Slovenia. Gynecol Obstet (Sunnyvale) 2016;6(350):2161-0932.
- 30. Evans P, Brunsell S. Uterine fibroid tumors: diagnosis and treatment. Am Fam Physician 2007 May;75(10):1503-1508.
- 31. Omole-Ohonsi A, Belga F. Surgical management of uterine fibroids at aminu kano teaching hospital. Obstet Gynecol Int 2012;2012:702325.
- 32. Ezeama C, Ikechebelu J, Obiechina Nj, Ezeama N. Clinical presentation of uterine fibroids in Nnewi, Nigeria: a 5-year review. Ann Med Health Sci Res 2012 Jul;2(2):114-118.
- 33. Zimmermann A, Bernuit D, Gerlinger C, Schaefers M, Geppert K. Prevalence, symptoms and management of uterine fibroids: an international internet-based survey of 21,746 women. BMC Womens Health 2012 Mar;12(1):6.
- 34. Chen I, Firth B, Hopkins L, Bougie O, Xie RH, Singh S. Clinical characteristics differentiating uterine sarcoma and fibroids. JSLS 2018 Jan-Mar;22(1):e2017.00066.
- 35. Eltoukhi HM, Modi MN, Weston M, Armstrong AY, Stewart EA. The health disparities of uterine fibroid tumors for African American women: a public health issue. Am J Obstet Gynecol 2014 Mar;210(3):194-199.
- 36. Al Sulaimani R, Machado L, Al Salmi M. Do large uterine fibroids impact pregnancy outcomes? Oman Med J 2021 Jul;36(4):e292.
- 37. Pavone D, Clemenza S, Sorbi F, Fambrini M, Petraglia F. Epidemiology and risk factors of uterine fibroids. Best Pract Res Clin Obstet Gynaecol 2018 Jan;46:3-11.
- 38. Bukulmez O, Doody KJ. Clinical features of myomas. Obstet Gynecol Clin North Am 2006 Mar;33(1):69-84.
- 39. Drayer SM, Catherino WH. Prevalence, morbidity, and current medical management of uterine leiomyomas. Int J Gynaecol Obstet 2015 Nov;131(2):117-122.
- 40. Shwayder J, Sakhel K. Imaging for uterine myomas and adenomyosis. J Minim Invasive Gynecol 2014 May-Jun;21(3):362-376.
- 41. McLucas B. Diagnosis, imaging and anatomical classification of uterine fibroids. Best Pract Res Clin Obstet Gynaecol 2008 Aug;22(4):627-642.
- 42. Shavell VI, Thakur M, Sawant A, Kruger ML, Jones TB, Singh M, et al. Adverse obstetric outcomes associated with sonographically identified large uterine fibroids. Fertil Steril 2012 Jan;97(1):107-110.
- 43. The World Bank Data. Fertility rate, total (births per woman). 2019 [cited 2022 March 29]. Available from: https://data.worldbank.org/indicator/SP.DYN.TFRT.IN?view=map.