Enzinger first described angiomatoid malignant fibrous histiocytoma (AFH) in 1979.1 It is a rare soft tissue tumor characterized by low malignant potential and rarely metastasizes. It accounts for 0.3% of all soft tissue sarcomas and is usually found in the deep dermis and subcutaneous tissues of the extremities of children and young adults.2 A few cases of AFH have been described in adults in literature.3,4 AFH has diverse clinicopathological features and is associated with specific genetic attributes.2 Because of its varied clinical presentation, it is difficult to establish a preoperative diagnosis of AFH. Here, we report a case of AFH in an elderly male patient.
Case report
A 65-year-old man presented in the surgery outpatient department at a rural tertiary care hospital in central India with complaints of swelling over the right leg lasting two months. It was insidious in onset and gradually progressive in nature. The patient gave a history of trauma to the right leg at the same site three months prior. There was no history of weight loss or decreased appetite. Physical examination was unremarkable. Local examination revealed a swelling measuring 7 × 4 cm over the anterolateral aspect of the right upper leg without tenderness.
Magnetic resonance imaging of the right leg revealed a well-defined lesion of the size 14.5 × 5.8 × 5.4 cm in an anterior intramuscular compartment in the proximal part of the right leg involving the tibialis anterior and right extensor digitorum longus muscles with multiple dilated and tortuous blood vessels in the subcutaneous plane. The lesion was abutting the right proximal tibia with thinning of its cortex, abutting and compressing anterior crural fascia, tibialis posterior muscle and its tendon, tibialis anterior vessels, and interosseous membrane [Figure 1]. A suspicion of soft tissue sarcoma was raised, and histopathological correlation was advised. Fine needle aspiration cytology was done from the site. It revealed low cellular smears, chiefly consisting of spindled cells arranged mostly singly with a high nuclear-to-cytoplasmic ratio, clumped chromatin, and prominent nucleoli [Figure 2]. The possibility of spindle cell sarcoma was raised.
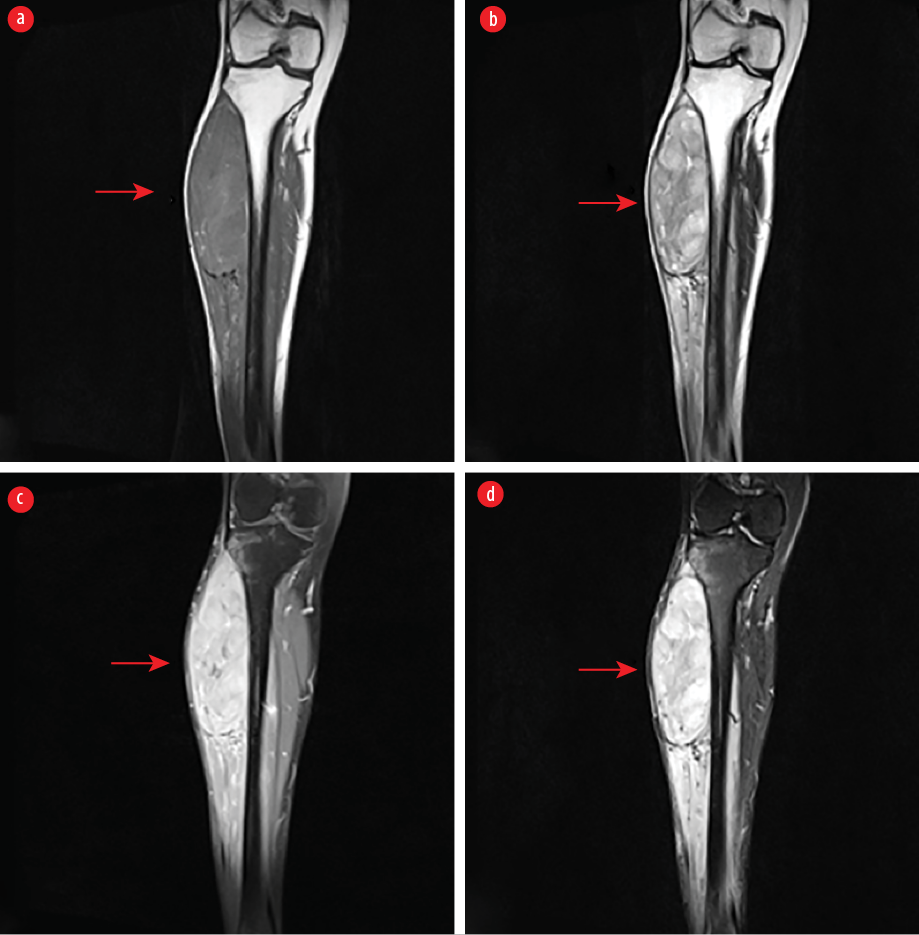 Figure 1: A well-defined spindle-shaped mass in the anterior intermuscular compartment of proximal right leg involving tibialis anterior and right extensor digitorum longus muscles. (a) The lesion shows intermediate signal intensity with few hyperintense foci on T1-weighted sequence, (b) heterogeneous hyperintensity on T2-weighted, (c) short Tau inversion recovery sequences, and (d) heterogenous post-contrast enhancement.
Figure 1: A well-defined spindle-shaped mass in the anterior intermuscular compartment of proximal right leg involving tibialis anterior and right extensor digitorum longus muscles. (a) The lesion shows intermediate signal intensity with few hyperintense foci on T1-weighted sequence, (b) heterogeneous hyperintensity on T2-weighted, (c) short Tau inversion recovery sequences, and (d) heterogenous post-contrast enhancement.
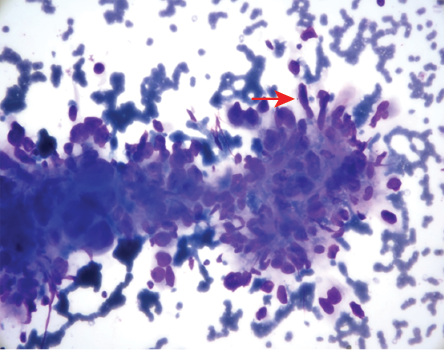 Figure 2: Fine needle aspiration cytology showing spindled cells (red arrow) with high nuclear-to-cytoplasmic ratio, clumped chromatin, and prominent nucleoli (Giemsa staining, magnification = 100 ×).
Figure 2: Fine needle aspiration cytology showing spindled cells (red arrow) with high nuclear-to-cytoplasmic ratio, clumped chromatin, and prominent nucleoli (Giemsa staining, magnification = 100 ×).
The patient underwent wide local surgical excision. A linear incision was taken over the lateral aspect of the right leg and deepened. An approximately 15 × 6 × 6 cm tumor mass was seen, probably originating from the tibialis anterior muscle and involving the extensor digitorum muscle. It extended to the tibia without any bone involvement. A sharp and blunt dissection with a 2 cm margin-wide local excision of the lesion was done. The remaining muscles were repaired. A drain was kept in situ, and closure was done in layers with compressive dressing. Grossly, the tumor was 17 × 6.5 × 3 cm. The cut surface showed gray-white to gray-red mass and was firm in consistency. Hematoxylin and eosin stained sections showed highly cellular tumor cells mixed with focal areas of hemorrhagic cyst-like spaces [Figure 3]. There was also the presence of abundant chronic inflammatory cells [Figure 4]. The individual tumor cells showed pleomorphism. The mitotic figures were 10 to 12 mitoses per 10 high-power fields. There was no evidence of vascular and neural invasion [Figure 5]. Immunohistochemistry (IHC) was done using formalin-fixed and paraffin-embedded tissue blocks. The tumor cells were found to be positive for vimentin (EP21, Rabbit Monoclonal Primary Antibody, Cell Marque) [Figure 6] and negative for desmin (EP15, 1:100, Rabbit Monoclonal Primary Antibody, Cell Marque) [Figure 7] and S100 (Anti-human S-100 protein, 15E2E2, Purified Bovine S-100 Protein, Biogenex) [Figure 8]. A diagnosis of AFH was made based on histopathology and IHC findings. The intraoperative and postoperative recovery of the patient was uneventful. The surgeon decided not to give this patient chemotherapy considering the intraoperative and histopathological findings. The patient remains under follow-up. There is no evidence of metastasis, and the patient is disease free three-years after excision.
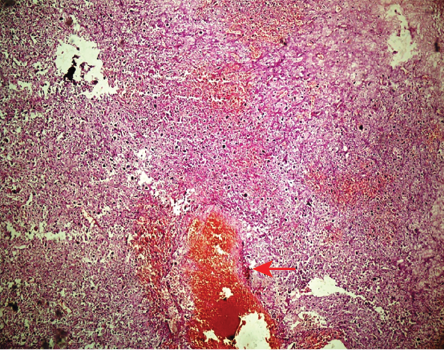 Figure 3: Section showing highly cellular tumor cells mixed with focal areas of hemorrhagic cyst-like spaces (red arrow) (Hematoxylin and eosin staining, magnification = 40 ×).
Figure 3: Section showing highly cellular tumor cells mixed with focal areas of hemorrhagic cyst-like spaces (red arrow) (Hematoxylin and eosin staining, magnification = 40 ×).
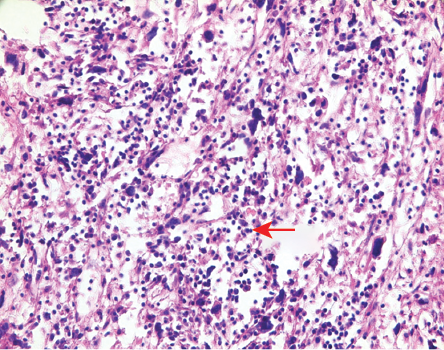 Figure 4: Section showing abundant chronic inflammatory cells (red arrow) (Hematoxylin and eosin staining, magnification = 100 ×).
Figure 4: Section showing abundant chronic inflammatory cells (red arrow) (Hematoxylin and eosin staining, magnification = 100 ×).
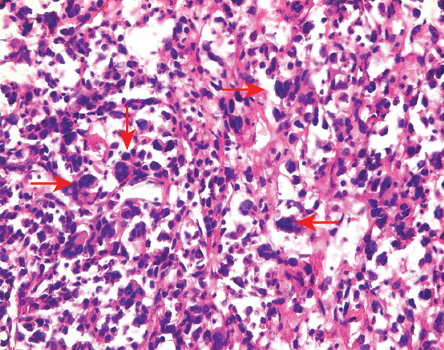 Figure 5: Section showing tumor cells with pleomorphism and mitoses (red arrows)
Figure 5: Section showing tumor cells with pleomorphism and mitoses (red arrows)
(Hematoxylin and eosin staining, magnification =
100 ×).
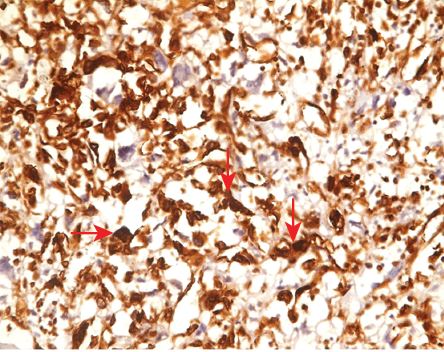 Figure 6: Positive staining for vimentin (magnification = 100 ×).
Figure 6: Positive staining for vimentin (magnification = 100 ×).
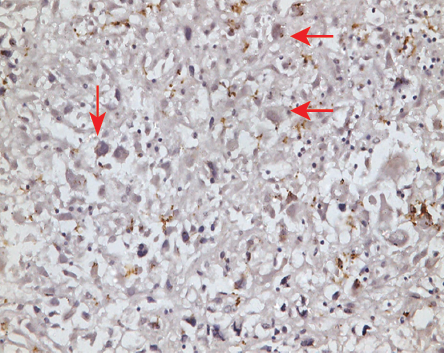 Figure 7: Negative staining for desmin (magnification = 100 ×).
Figure 7: Negative staining for desmin (magnification = 100 ×).
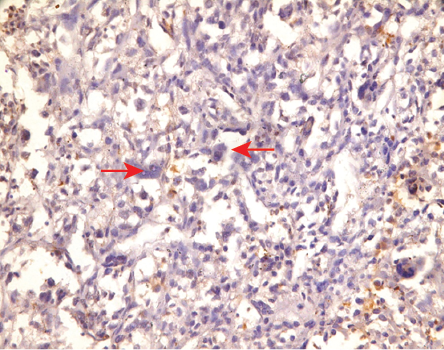 Figure 8: Negative staining for S-100 (magnification = 100 ×).
Figure 8: Negative staining for S-100 (magnification = 100 ×).
Discussion
AFH is classified as an intermediate tumor.2 It was initially considered a variant of malignant fibrous histiocytoma, but a follow-up study of 108 cases showed a favorable prognosis, which ultimately led to its recognition as a distinct entity.5 AFH is no longer regarded as “malignant” in the present era as the precise line of differentiation is still unknown. Thus, the entity has now been removed as a subtype of malignant sarcoma and is placed under the category of “intermediate tumors of uncertain potential” according to the 2013 World Health Organization classification.2 The exact line of differentiation for AFH remains unknown.2 The literature shows reports of AFH in children and young adults and very rarely in adults.2–5 The median age for presentation is 21 years, with a slight male preponderance. Ajlan et al,3 reported two cases of AFH in 28- and 85-year-old patients while Saito et al,4 found ages ranging from 8 to 50 years old in a study of seven cases. Extremities are the commonest location of occurrence for AFH. Other extrasomatic soft tissue locations include the mediastinum, lung, retroperitoneum, ovary, lung, bone, and brain.6
Grossly, the tumor measures around 2–4 cm; however, few studies also showed large-sized AFH.7 The mass is usually firm with hemorrhagic cystic spaces.2 Microscopically, AFH shows the presence of a fibrous pseudocapsule, round to spindled fibrohistiocytic cell proliferation, a plasmalymphocytic infiltrate, and a pseudoangiomatous pattern.2,7 The presence of hemorrhagic cystic spaces cannot be explained in these tumors as clinically patients do not have either thrombocytopenia or any defect in the coagulation system.6 Morphologically, there can be presence of solid or cellular variants of AFH. The differential diagnoses for AFH include myxoid tumors (like extraskeletal myxoid chondrosarcoma), low-grade fibromyxoid sarcoma, and myxoid liposarcoma. These can be differentiated by the absence of myxoid material, rhadbdomyoblasts, and myxoid spindle cells on morphology. There are no specific IHC markers for the exact diagnosis of AFH. Around 50% of these tumors express desmin.4 Epithelial membrane antigen is usually positive. It can be positive for a few other myxoid markers like calponin, smooth muscle actin, or rarely h-caldesmon but negative for skeletal markers like myogenin.4 Our case was positive for vimentin and negative for desmin and S100.
Molecular studies, like FISH, help to detect the rearrangement of EXSR1-ATF-1 or FUS-ATF1 fusion transcripts.6 Tanas et al,8 reported that 76% of cases of AFH in their study were diagnosed by FISH to harbor EWSR1 rearrangement. It helps to confirm the diagnosis of AFH in cases where typical morphological and IHC features are absent. These cases are usually treated with surgical excision with a wide local excision.9,10 However, radiation therapy is utilized in cases where marginal excision is planned depending on the surgeon’s point of view. The literature shows different recurrence and metastasis rates in these cases. Enzinger,1 found local recurrence and metastasis in 63% and 21% of cases, while Pettinato et al,11 found recurrence in 25% and metastasis in 5% of cases. In our case, there is no evidence of metastasis or recurrence three years after excision.
The majority of AFH cases are present in the second and third decades of life. Very few cases have been reported in the elderly age group like that of our case to the best of our knowledge.
Conclusion
AFH is an uncommon soft tissue tumor that can be found even in the elderly. The lack of specific clinical manifestations and radiological and histopathological features makes the diagnosis more difficult. Treatment includes appropriate surgical excision to obtain negative surgical margins, and it is essential to keep the patient under follow-up to look for any clinical evidence of tumor recurrence.
Disclosure
The authors declared no conflicts of interest. Informed consent has been obtained from the patients.
references
- 1. Enzinger FM. Angiomatoid malignant fibrous histiocytoma: a distinct fibrohistiocytic tumor of children and young adults simulating a vascular neoplasm. Cancer 1979 Dec;44(6):2147-2157.
- 2. Antonescu C, Rossi S. Angiomatoid fibrous histiocytoma. In: Fletcher CDM, Bridge JA, Hogendoorn PC, Mertens F, editors. WHO classification of tumors of soft tissue and bone. 4th ed. Lyon: IARC; 2013. p. 204-205.
- 3. Ajlan AM, Sayegh K, Powell T, David H, Riha RM, Khan J, et al. Angiomatoid fibrous histiocytoma: magnetic resonance imaging appearance in 2 cases. J Comput Assist Tomogr 2010 Sep-Oct;34(5):791-794.
- 4. Saito K, Kobayashi E, Yoshida A, Araki Y, Kubota D, Tanzawa Y, et al. Angiomatoid fibrous histiocytoma: a series of seven cases including genetically confirmed aggressive cases and a literature review. BMC Musculoskelet Disord 2017 Jan;18(1):31.
- 5. Costa MJ, Weiss SW. Angiomatoid malignant fibrous histiocytoma. A follow-up study of 108 cases with evaluation of possible histologic predictors of outcome. Am J Surg Pathol 1990 Dec;14(12):1126-1132.
- 6. Rekhi B, Adamane S, Ghodke K, Desai S, Jambhekar NA. Angiomatoid fibrous histiocytoma: Clinicopathological spectrum of five cases, including EWSR1-CREB1 positive result in a single case. Indian J Pathol Microbiol 2016 Apr-Jun;59(2):148-152.
- 7. Fletcher CD, Bridge JA, Hogendoorn PC, Mertens F. WHO classification of tumours of soft tissue and bone. 4th ed. Lyon: IARC Press; 2013.
- 9. Tanas MR, Rubin BP, Montgomery EA, Turner SL, Cook JR, Tubbs RR, et al. Utility of FISH in the diagnosis of angiomatoid fibrous histiocytoma: a series of 18 cases. Mod Pathol 2010 Jan;23(1):93-97.
- 10. Huerter ME, Hammadeh R, Zhou Q, Riker AI. Recurrent angiomatoid fibrous histiocytoma: a case report and review of the literature. Ochsner J 2014;14(3):441-444.
- 11. Hashimoto K, Nishimura S, Kakinoki R, Akagi M. Treatment of angiomatoid fibrous histiocytoma after unplanned excision: a case report. BMC Res Notes 2018 Aug;11(1):628.
- 12. Pettinato G, Manivel JC, De Rosa G, Petrella G, Jaszcz W. Angiomatoid malignant fibrous histiocytoma: cytologic, immunohistochemical, ultrastructural, and flow cytometric study of 20 cases. Mod Pathol 1990 Jul;3(4):479-487.