COVID-19 is a severe respiratory disease caused by the novel SARS-CoV-2, which emerged in December 2019.1 Individuals with a mild form of the disease have an efficient immune system controlling and eliminating the virus. On the other hand, individuals with a severe form of illness experience intense immune responses that may result in multiple organ pathology. Therefore, patients may develop acute kidney injury, coagulopathy, cerebrovascular events, and septic shock.2,3
Case report
A 36-year-old male had warm autoimmune hemolytic anemia (WAIHA) three-month post mild-COVİD-19 infection that did not require hospitalization and was treated conservatively. He presented with a one-week history of anemia symptoms including fatigue, tiredness, shortness of breath, back pain, and tea-colored urine. His medical history was negative for recent ingestion of fava beans or medications. Further, he had no family history suggestive of congenital hemolytic anemia.
Clinical examination revealed tachycardia at 105/min and marked jaundice in the sclera. Investigations revealed normocytic anemia with hemoglobin levels of 9.5 g/dL, and thrombocytopenia with a platelet count of 121 × 109/dL. Additionally, we observed reticulocytosis, indirect hyperbilirubinemia, and normal liver enzymes with no evidence of disseminated intravascular coagulation. Peripheral smear revealed spherocytes and polychromasia, with a few red blood cell fragments, but no agglutination or red blood cell clumping could be seen. Both direct and indirect Coombs tests were positive. He tested negative for HIV, hepatitis B virus, and hepatitis C virus during a recent employment assessment.
A cross-sectional imaging of the body with contrast was normal. The clinical and laboratory findings were consistent with immunoglobulin G WAIHA. The patient was initially started on 1 mg/kg prednisone and escalated to 1 g methylprednisolone for three consecutive days. However, due to a lack of response and further deterioration in hemoglobin levels, weekly rituximab in four divided doses was added to the treatment protocol. On day 10, the patient had a significant resolution of the clinical symptoms and was discharged from the hospital following the second dose of rituximab. He had an excellent response to treatment in the form of substantial improvement in hemoglobin level and regression of hemolysis parameters [Figure 1].
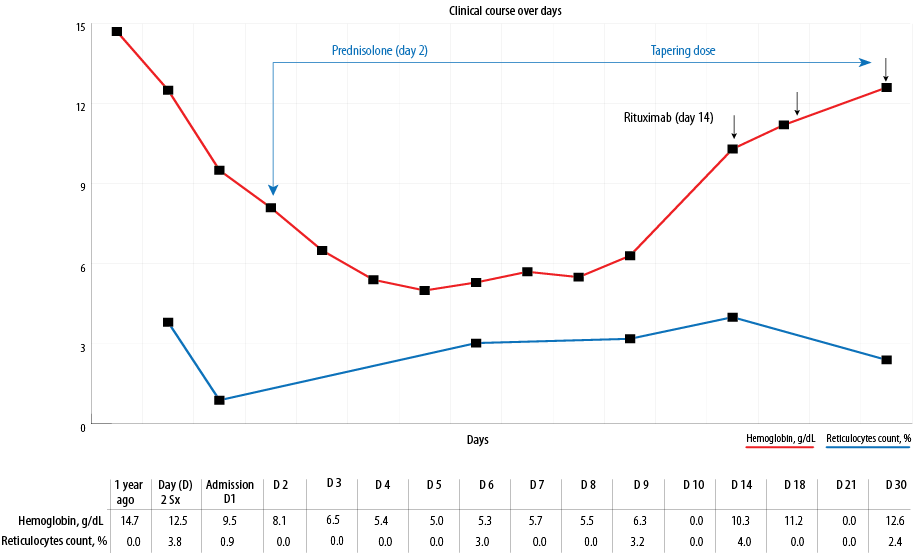 Figure 1: Illustration of the clinical course of the disease from the time of diagnosis until complete recovery.
Figure 1: Illustration of the clinical course of the disease from the time of diagnosis until complete recovery.
Discussion
WAIHA is an acquired heterogeneous hemolytic disorder caused by the host’s immune system acting against red cell antigens. WAIHA can be idiopathic in around 45% of cases. Secondary causes of WAIHA include autoimmune disease, lymphoproliferative disorders, and miscellaneous causes, including infections and drugs.4,5 Glucocorticoids are considered the first-line treatment. While splenectomy is historically the treatment of choice in refractory cases, rituximab increasingly superseded it.6
Several reports indicate that COVID-19 has been associated with several autoimmune phenomena. WAIHA has been increasingly recognized in patients with COVID-19 as an initial presentation or shortly after the infection. The clinical course of WAIHA-related COVID-19 may range from a quiescent course where no treatment is needed to a refractory course requiring more than one line of treatment.7,8 Unlike previous reports, our patient presented with features of WAIHA as a late presentation for COVID-19. Moreover, he was resistant to the steroid’s pulse dose that mandated the initiation of monoclonal antibody medication in the form of rituximab, similar to the previously described reports.9,10
The result from this report reflects the importance of serial follow-up of complete blood cell count parameters and hemolysis markers post-COVID-19 infections. Additionally, WAIHA post-COVID-19 infection might be more resistant to standard treatment that warrants second treatment line, such as rituximab. However, a time frame of at least six months following rituximab infusion is needed before vaccination becomes effective.11 This raises the question of whether refractory cases of WAIHA should be treated differently in the COVID-19 era while awaiting coronavirus vaccination.
Conclusion
WAIHA is a frequently reported complication in patients with COVID-19 disease with a broad spectrum of clinical courses and variable responses to treatment.
Disclosure
The authors declared no conflicts of interest. Informed consent was obtained from the patient.
references
- 1. Poland GA, Ovsyannikova IG, Kennedy RB. SARS-CoV-2 immunity: review and applications to phase 3 vaccine candidates. Lancet 2020 Nov;396(10262):1595-1606.
- 2. de Oliveira AP, Lopes AL, Pacheco G, de Sá Guimarães Nolêto IR, Nicolau LA, Medeiros JV. Premises among SARS-CoV-2, dysbiosis and diarrhea: Walking through the ACE2/mTOR/autophagy route. Med Hypotheses 2020 Nov;144:110243.
- 3. Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA 2020 Aug;324(8):782-793.
- 4. Hill A, Hill QA. Autoimmune hemolytic anemia. Hematology Am Soc Hematol Educ Program 2018 Nov;2018(1):382-389.
- 5. Jäger U, Barcellini W, Broome CM, Gertz MA, Hill A, Hill QA, et al. Diagnosis and treatment of autoimmune hemolytic anemia in adults: recommendations from the first international consensus meeting. Blood Rev 2020 May;41:100648.
- 6. Go RS, Winters JL, Kay NE. How I treat autoimmune hemolytic anemia. Blood 2017 Jun;129(22):2971-2979.
- 7. Jacobs J, Eichbaum Q. COVID-19 associated with severe autoimmune hemolytic anemia. Transfusion 2020;2(March):2-7.
- 8. Campos-Cabrera G, Mendez-Garcia E, Mora-Torres M, Campos-Cabrera S, Campos-Cabrera V, Garcia-Rubio G, et al. Autoimmune hemolytic anemia as initial presentation of COVID-19 infection. Blood 2020;136(Supplement 1);136:8.
- 9. Lazarian G, Quinquenel A, Bellal M, Siavellis J, Jacquy C, Re D, et al. Autoimmune haemolytic anaemia associated with COVID-19 infection. Br J Haematol 2020 Jul;190(1):29-31.
- 10. Jawed M, Hart E, Saeed M. Haemolytic anaemia: a consequence of COVID-19. BMJ Case Rep 2020 Dec;13(12):10-13.
- 11. Houot R, Levy R, Cartron G, Armand P. Could anti-CD20 therapy jeopardise the efficacy of a SARS-CoV-2 vaccine? Eur J Cancer 2020 Sep;136:4-6.