Albinism is a heterogeneous group of inheritable autosomal recessive disorders characterized by lacking or defective tyrosinase enzyme (which is required to convert tyrosine to the melanin precursor dioxyphenylalanine) leading to reduced or absent melanin production in the melanocytes of the skin, eyes, and hair.1 Albinism can occur in syndromic and non-syndromic forms.2 The global incidence of albinism is 1:20 000 individuals, with a higher prevalence in sub-Saharan Africa, ranging from 1:15 000 to 1:1000.2 According to the Albino Foundation, there are about two million albinos in Nigeria.3 The lack or dysfunctional state of melanin in the albinos exposes them to the deleterious effects of ultraviolet radiation, predisposing them to cutaneous and ocular pathologic conditions4 including skin cancers such as squamous cell carcinoma and basal cell carcinoma.5
Primary carcinosarcoma of the skin (aka metaplastic carcinoma, biphasic sarcomatoid carcinoma, and malignant mixed tumor) is an extremely rare biphasic tumor consisting of an intimate admixture of epithelial and heterologous mesenchymal elements, both of which are malignant.6,7 The histogenesis remains unclear. The most widely accepted theory is the monoclonal hypothesis which postulates the divergence of a single totipotent stem cell into separate epithelial and mesenchymal directions.8 Another view is that the sarcomatous component of the tumor is a metaplastic transformation of the carcinomatous component.6,9 Some authors have suggested the possibility of a collision between two different tumors or a pseudosarcomatous reaction within the epithelial malignancy.10 Only about 120 cases of cutaneous carcinosarcoma have been reported to date in the literature.11 To our knowledge, it has not been reported in an African albino. Here, we present the index case of a primary carcinosarcoma of the skin in a Nigerian albino woman.
Case report
A 57-year-old female albino patient of African ancestry presented with a year’s history of multiple skin lesions. She had an ulcerated tumor (5 × 5 cm) on the nasal bridge extending to the medial canthal region [Figure 1], a globular pedunculated growth (10 × 8 × 8 cm) hanging from a stalk on the right side of the neck and ulcerated lesions on the right forearm and upper back. There were no palpable lymph nodes. Review and examination of other systems were normal.

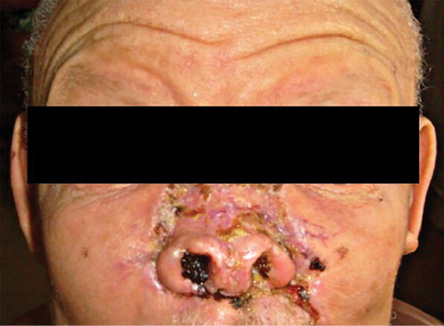 Figure 1: An ulcerated tumor on the nasal bridge extending to the medial canthal region.
Figure 1: An ulcerated tumor on the nasal bridge extending to the medial canthal region.

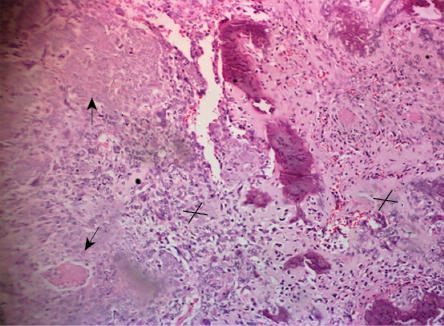 Figure 2: Histology section showing a biphasic malignant tumor (hematoxylin and eosin stain, magnification = 50 ×).
Figure 2: Histology section showing a biphasic malignant tumor (hematoxylin and eosin stain, magnification = 50 ×).

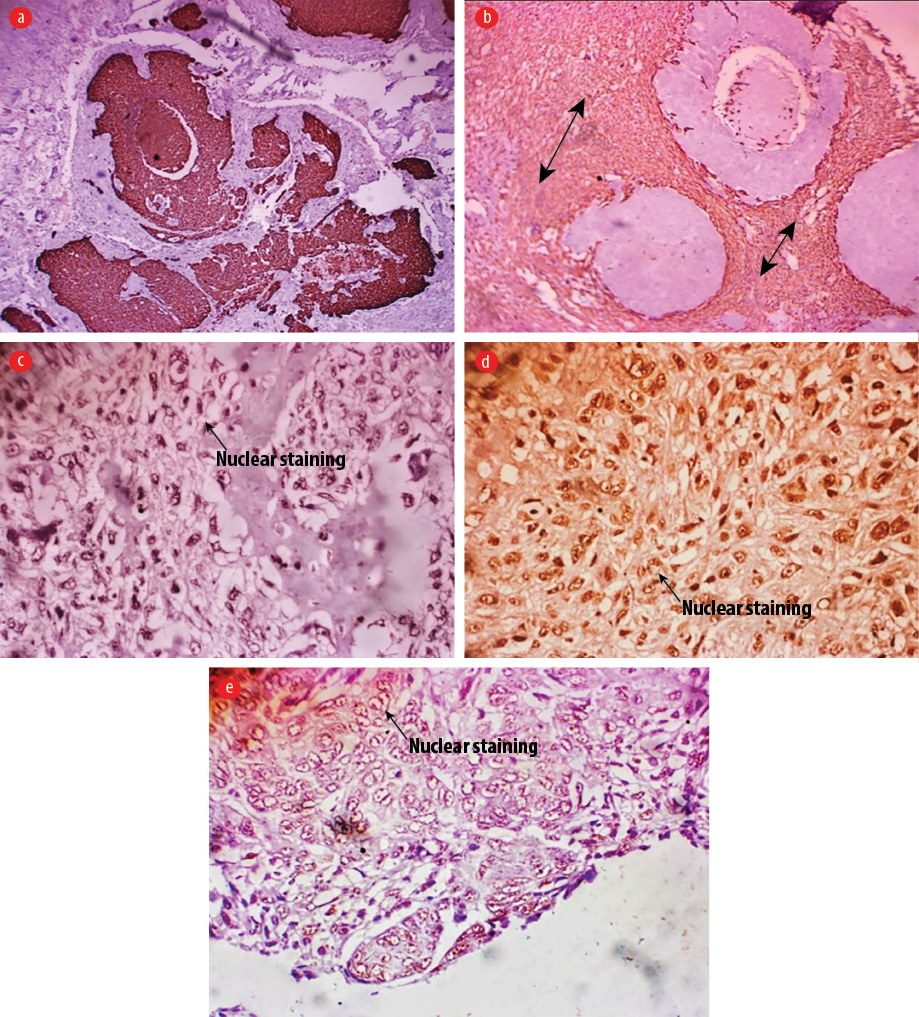 Figure 3: Immunohistochemistry images. (a) Only the epithelial component was positive (cytoplasmic) for pancytokeratin immunohistochemical stain, magnification = 50 ×), while (b) only the sarcomatous component was positive for vimentin (vimentin immunohistochemical stain, magnification = 50 ×).
Figure 3: Immunohistochemistry images. (a) Only the epithelial component was positive (cytoplasmic) for pancytokeratin immunohistochemical stain, magnification = 50 ×), while (b) only the sarcomatous component was positive for vimentin (vimentin immunohistochemical stain, magnification = 50 ×).
(c–e) Both the epithelial and sarcomatous components were positive for p53 protein immunohistochemical staining, 100 × magnification.
Three years prior to presentation, the patient underwent a wide local excision with tumor-free margins and grafting for similar lesions diagnosed as squamous cell carcinoma. She did not undergo irradiation as part of that treatment.
A chest X-ray and abdominopelvic ultrasound were performed to rule out visceral primary. Computed tomography scan and magnetic resonance imaging could not be conducted due to financial constraint. Ancillary laboratory investigations were normal except for mild anemia.
Histology sections [Figure 2] showed a biphasic malignant tumor arising from the overlying epidermis. It was composed of atypical cells having hyperchromatic, vesicular nuclei and moderate cytoplasm. These cells were disposed in nests, trabeculae, cords, and adenoid pattern, and intimately admixed with malignant stroma having atypical pleomorphic spindle cells. There were osteoid and cartilage formations within the malignant stroma. There was high mitotic activity with a count of 25 per 10 high power fields including abnormal forms. There was no evidence of neural or vascular invasion. Immunohistochemistry results [Figure 3] showed the epithelial component to be positive for pancytokeratin and p53 protein, while the sarcomatous component was positive for vimentin and p53.
A diagnosis of primary carcinosarcoma of the skin was made. The patient was admitted and had an excisional biopsy of the lesions. Wound care for the skin lesions following wide excision included alternate-day wound dressing with Sofra-tulle gauze (gauze dressing impregnated with the antibiotic framycetin sulfate). No grafting was done. The patient was followed-up for six months post-surgery and had a good outcome. One year post-excision, no recurrence was noted.
Discussion
Skin cancer is rare among Africans, and albinism is an established risk factor for skin cancer in this population.12 Albinism is a genetically inherited disorder with a worldwide distribution. Phenotypically, it presents with reduced or no melanin in the hair, skin, and eyes. African albinos living near the equator (where ultraviolet radiation is high) have the highest risk of skin cancers on the continent. Albinos are at higher risk of developing malignant skin lesions at younger ages than the general population, usually from the third decade of life.12 The usual culprits are squamous cell carcinoma, basal cell carcinoma, and malignant melanoma,12 as well as basosquamous carcinoma and collision of squamous cell carcinoma and basal cell carcinoma.13
Our patient was diagnosed with carcinosarcoma at the age of 57 years, earlier than the reported mean age of 71.5 years.11 Case series by Kwak et al,11 and Clark et al,14 showed a male preponderance.Microscopically, the more common carcinoma component is a squamous cell carcinoma followed by basal cell carcinoma, whereas the most common sarcomatous component is osteosarcoma.6 Other malignant heterologous mesenchymal elements include chondrosarcoma, leiomyosarcoma, rhabdomyosarcoma, fibrosarcoma, angiosarcoma, and giant cell tumor of soft parts.7 Our patient's case is an admixture of squamous cell carcinoma and sarcoma with osteosarcomatous differentiation.
Cutaneous carcinosarcoma is broadly classified into two distinct groups.6,15 Epidermal-derived (basal or squamous cell carcinoma epithelial component with a sarcomatous component) carcinosarcomas arise on the sun-damaged skin of the head and neck of elderly males (mean age = 72 years) with a 70% probability of five-year disease-free survival.6 In contrast, adnexal carcinosarcomas (spiradenocarcinoma, porocarcinoma, proliferating trichilemmal cystic carcinoma, or matrical carcinoma) present as recent growth in a long-standing nodule. They occur in younger patients (mean age = 58 years) with 25% five-year disease-free survival odds.6
Immunoperoxidase staining are of great value in elucidating the components of the tumors as in the present case. While the cytokeratin marker AE1/AE3 may be negative in the epithelial component, p63 and MNF116 are often expressed in poorly differentiated epithelial cells.7 In cases with basaloid epithelial component (basal cell carcinosarcomas), markers such as BerEP4 and Bcl-2 have also shown positivity.16 In addition, p53 has been reported to be overexpressed by both epithelial and mesenchymal components.16 However, the case series by Kwak et al,11 showed such co-expression in only two of the 11 cases, with no expression in either component in one case, while four cases each had p53 expression in either the epithelial or mesenchymal component.The mesenchymal component may express vimentin, actin, CD10, CD34, and CD68, depending on its differentiation.7 There was co-expression of p53 by the carcinomatous and sarcomatous elements in the index case.
Differential diagnoses include spindle cell squamous cell carcinoma and biphasic synovial sarcoma. Spindle cell squamous cell carcinoma consists of malignant spindle cells with variable components of invasive squamous cell carcinoma.16 If spindle cells are not clearly segregated to epithelial or mesenchymal components with expression of cytokeratin with or without p63, they are confirmed as spindle cell squamous cell carcinoma rather than carcinosarcoma.14,16 Up to 40% of spindle cell squamous cell carcinomas express vimentin. Therefore, it is difficult to use vimentin in carcinosarcoma for diagnostic purposes.16,17 However, vimentin can provide additional information in differentiating the two neoplastic components of carcinosarcoma.16,17 Biphasic synovial sarcoma consists of cord, nest, or gland-shaped epithelial cells and spindle cells with expression of TLE1 or EMA in both cell types.14,16
In contrast to metaplastic carcinomas arising in visceral sites, those primarily arising in the skin do not appear to behave in a very aggressive manner.6,15 Complete excision of the lesion with 10 mm free circumferential margin is the treatment of choice; adjuvant chemotherapy or radiotherapy has not been shown to be beneficial.8 The patient had a wide local excision and has had no recurrence more than a year since then.
Conclusion
We have presented a rare case of primary cutaneous carcinosarcoma in a Nigerian albino following recurrent cutaneous squamous cell carcinomas. Furthermore, we advocate increased enlightenment of albinos, especially those in Africa, on the use of sunscreens to prevent the incidence of skin malignancies among them.
Disclosure
The authors declared no conflicts of interest. Written consent was obtained from the patient.
references
- 1. Oetting WS, King RA. Molecular basis of oculocutaneous albinism. J Invest Dermatol 1994 Nov;103(5)(Suppl):131S-136S.
- 2. Marçon CR, Maia M. Albinism: epidemiology, genetics, cutaneous characterization, psychosocial factors. An Bras Dermatol 2019 Sep - Oct;94(5):503-520.
- 3. The Albino Foundation. Albinism in Africa. [cited 2020 October 10]. Available from: http://Albino foundation.org/albinism/albinism in Africa.
- 4. Andres E. Cruz-Inigo, MDa, Barry Ladizinski, MDb, Aisha Sethi. Albinism in Africa: stigma, slaughter and awareness campaigns. Dermatol Clin 2011;29:79-87.
- 5. Awe OO, Azeke TA. Cutaneous cancers in Nigerian albinos: A review of 22 cases. Niger J Surg 2018 Jan-Jun;24(1):34-38.
- 6. Moore JB, Ragsdale BD. Tumors with fatty, muscular, osseous, and/or cartilaginous differentiation. In: Elder DE, Elenitsas R, Rosenbach M, Murphy GF, Rubin AI, Xu X (eds). Lever’s Histopathology of the Skin. Wolters Kluwer Health: Philadelphia; 2015. p. 1358.
- 7. Patterson JW. Tumors of the epidermis. In: Weedon’s Skin Pathology. Elsevier; 2016. p. 784-835.
- 8. Liaw T-Y. Primary cutaneous sarcomatoid carcinoma presenting as a rapidly-growing and ulcerative tumor of the skin. Kaohsiung J Med Sci 2017 Jun;33(6):315-317.
- 9. Patel NK, McKee PH, Smith NP, Fletcher CD. Primary metaplastic carcinoma (carcinosarcoma) of the skin. A clinicopathologic study of four cases and review of the literature. Am J Dermatopathol 1997 Aug;19(4):363-372.
- 10. Luong TM, Akazawa Y, Mussazhanova Z, Matsuda K, Ueki N, Miura S, et al. Cutaneous pilomatrical carcinosarcoma: a case report with molecular analysis and literature review. Diagn Pathol 2020 Jan;15(1):7.
- 11. Kwak HB, Park J, Kim HU, Nam KH, Yun SK. Cutaneous carcinosarcoma: a clinicopathologic and immunohistochemical analysis of 11 korean cases. J Korean Med Sci 2018 Dec;34(1):e5.
- 12. Kiprono SK, Chaula BM, Beltraminelli H. Histological review of skin cancers in African Albinos: a 10-year retrospective review. BMC Cancer 2014 Mar;14:157.
- 13. Enechukwu NA, Ogun GA, Ezejiofor OI, Chukwuanukwu TO, Yaria J, George AO, et al. Histopathologic patterns of cutaneous malignancies in individuals with oculocutaneous albinism in Anambra state, Nigeria: a paradigm swing? Ecancermedicalscience 2020;14:1013.
- 14. Clark JJ, Bowen AR, Bowen GM, Hyngstrom JR, Hadley ML, Duffy K, et al. Cutaneous carcinosarcoma: a series of six cases and a review of the literature. J Cutan Pathol 2017 Jan;44(1):34-44.
- 15. Tran TA, Muller S, Chaudahri PJ, Carlson JA. Cutaneous carcinosarcoma: adnexal vs. epidermal types define high- and low-risk tumors. Results of a meta-analysis. J Cutan Pathol 2005 Jan;32(1):2-11.
- 16. Lim Y, Byun HJ, Park CS, Lee JH, Park JH, Lee JH, et al. Primary cutaneous carcinosarcoma developing after chronic C-arm radiation exposure. JAAD Case Rep 2018 Jan;4(2):126-128.
- 17. Müller CS, Pföhler C, Schiekofer C, Körner R, Vogt T. Primary cutaneous carcinosarcomas: a morphological histogenetic concept revisited. Am J Dermatopathol 2014 Apr;36(4):328-339.