Salivary gland tumors are a rare cause of tracheobronchial tumors. The most common type, mucoepidermoid carcinoma (MEC), generally presents with symptoms of airway obstruction. They are a rare cause of lung tumors, and it is important to differentiate them from other more aggressive tumors.1,2
We present a case of a young lady with MEC who was initially mistakenly diagnosed with adenocarcinoma of the lung. It is important to correlate biopsy results with the clinical picture because of possible diagnostic limitations.
Case report
A 43-year-old woman with no risk factors presented with recurrent episodes of productive cough and fever for one year. Her sputum was white but occasionally green without blood. Her cough was associated with outbreaks of fever and malaise. She was treated several times with antibiotics. She was not a smoker, had no exposure to tuberculosis and animals, and denied prior travel.
Computed tomography (CT) scan showed a mass in the right main bronchus, right upper lobe collapse, and enlarged mediastinal lymph nodes [Figure 1]. Endobronchial tumor of unknown type was suspected.
She then had a bronchoscopic biopsy and positron emission tomography (PET) scan. Biopsy was reported as adenocarcinoma of the lung. PET scan showed uptake only in the mass with no distant disease.

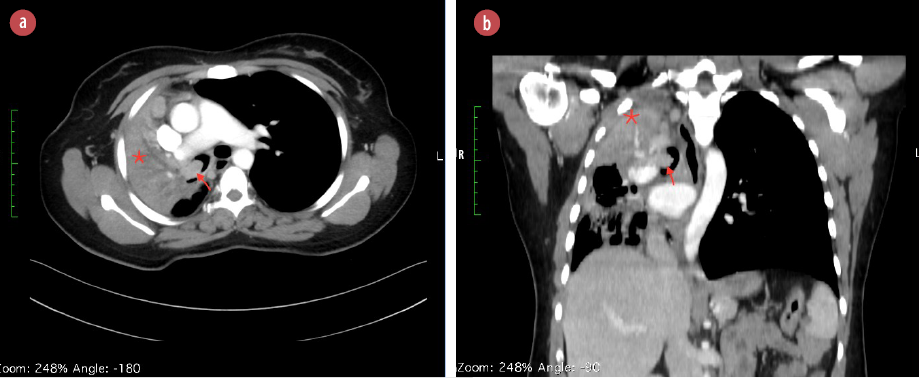 Figure 1: CT scan of the chest with (a) axial and (b) sagittal views demonstrating an endobronchial mass (red arrows) and collapse of the right upper and middle lobe (*). The images also demonstrate mediastinal shift to the right side and mediastinal lymph node enlargement.
Figure 1: CT scan of the chest with (a) axial and (b) sagittal views demonstrating an endobronchial mass (red arrows) and collapse of the right upper and middle lobe (*). The images also demonstrate mediastinal shift to the right side and mediastinal lymph node enlargement.

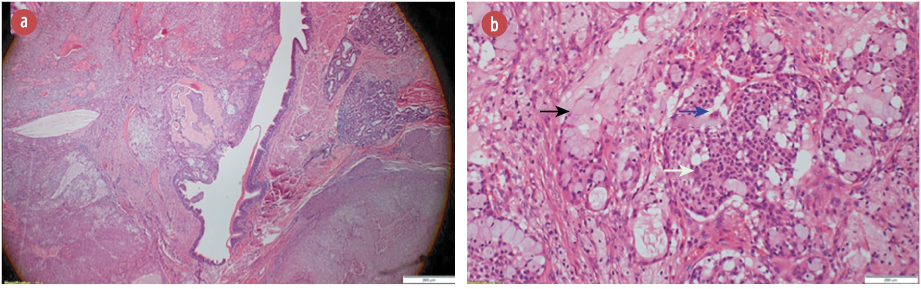 Figure 2: (a) Hematoxylin and eosin stain showing a polypoid tumor protruding into the bronchial lumen, magnification = 4 ×. (b) Hematoxylin and eosin stain showing tumor composed of a mixture of mucin secreting cells (black arrow), admixed with sheets of squamoid (white arrow), and intermediate cells (blue arrow) magnification = 40 ×. These cells lack significant nuclear atypia, mitotic activity, and necrosis.
Figure 2: (a) Hematoxylin and eosin stain showing a polypoid tumor protruding into the bronchial lumen, magnification = 4 ×. (b) Hematoxylin and eosin stain showing tumor composed of a mixture of mucin secreting cells (black arrow), admixed with sheets of squamoid (white arrow), and intermediate cells (blue arrow) magnification = 40 ×. These cells lack significant nuclear atypia, mitotic activity, and necrosis.

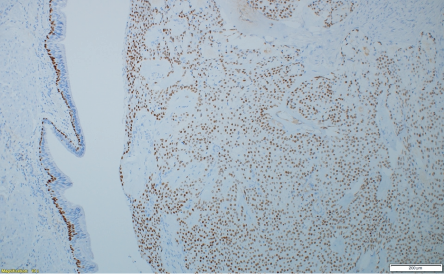 Figure 3: P63 immunohistochemistry stain at showing tumor cells with strong nuclear positivity, magnification = 10 ×.
Figure 3: P63 immunohistochemistry stain at showing tumor cells with strong nuclear positivity, magnification = 10 ×.
The patient then presented to our center. She only had a report indicating a diagnosis of adenocarcinoma of the lung. Slides were not obtainable as the biopsy was done abroad. Since the mediastinal lymph nodes were significantly large, they were biopsied and showed reactive hyperplasia with no evidence of malignancy.
She was staged as T2N0M0 adenocarcinoma of the lung. The plan was to surgically resect the tumor with lobectomy and mediastinal lymph node dissection (MLND).
An intraoperative bronchoscope was done by the surgeon. It showed a smooth mass occupying the right upper lobe bronchus bulging into the right main bronchus. It did not have the typical appearance of bronchial adenocarcinoma. This appearance, together with the very indolent, slow clinical history and negative large mediastinal lymph nodes, raised doubts about whether the diagnosis of adenocarcinoma was correct.
A right posteriorlateral thoracotomy was performed. The right upper lobe parenchyma appeared very normal and not diseased. The right upper and right main bronchus were cut open, exposing the tumor and allowing it to be resected en-block. It was decided to defer lobectomy as planned and wait for the final pathology as there was no doubt regarding the diagnosis of adenocarcinoma. The introoperative frozen section of the margins came back negative for malignancy. Therefore, sleeve anastomosis of the right lobe bronchi to the main bronchus with preservation of the lung parenchyma was performed. The anastomosis was reinforced with an intercostal muscle flap. Finally, MLND was done.
Her postoperative course was uneventful. At follow-up, she was doing well, and the right lower lobe was fully expanded on chest X-ray.
The final histopathology was surprising. It reported a 35 mm malignant neoplasm. The lesion was composed of solid sheets and nests of neoplastic squamous, intermediate, and mucus-secreting cells with few cystic changes [Figure 2a]. Mitotic count was < 1 per 10 high-powered fields [Figure 2b]. Immunohistochemistry stains were positive for p63, CK5/6, and CK7 but negative for napsin, TTF1, and PD-L1 [Figure 3]. Impression was a low-grade MEC with no evidence of adenocarcinoma. Lymph nodes were negative for metastasis. The final staging was T2aN0M0.
Discussion
Salivary gland tumors typically occur in the head and neck region but rarely in the trachea, lung, and skin.3 In the pulmonary system, it accounts for < 1% of primary pulmonary tumors.4 They arise from the bronchial glands4,5 and can be divided into three subtypes: MEC, adenoid cystic carcinoma (ACC), and epithelial-myoepithelial carcinoma (EMC), where MEC is the most common.
Patients with tracheobronchial MEC (TMEC) clinically present with symptoms caused by airway obstruction.3,6 MEC is not associated with high-risk behaviors such as smoking and industrial exposures;1 70.7% of MEC are centrally located, smooth and well-circumscribed, consisting of solid and cystic components.2,6,7 Histologically, they are a mixture of mucus-producing, glandular and squamous epithelial cells, intermediate cells at various percentages, and various growth patterns such as cystic, papillary, and solid. They stain positive for CK5/6, CK7, p53, and p40.
Contrarily, adenocarcinomas are located in the periphery, and cells are arranged in an acinar pattern and stain positive for CK7, TTF-1, and napsin A.3 In a small biopsy, the spectrum of the typical histomorphology may not be present, making diagnosis difficult.
High-grade TMEC can be difficult to distinguish from adenosquamous carcinoma. Yousem et al,2 proposed differentiation criteria. In TEMC, surface epithelium does not show carcinoma-in-situ, and although squamous metaplasia may be present, atypical metaplasia is not present except coincidently. Cells of both solid and glandular areas have a more uniform, almost bland appearance; unlike bronchogenic carcinoma, nuclear features and cytoplasmic variability are pronounced. Large areas of frank keratinization, including abundant individual cell keratinization, is a feature of bronchogenic carcinoma that is not seen in TEMC.
Despite these criteria, significant overlap exists, and final diagnosis may not be possible until the final surgical resection. As such, in this case, when the clinical history, gross appearance, and overall pattern of the disease do not concede with a more aggressive pathology, it is not unreasonable to embark on a less aggressive surgery until the final diagnosis is confirmed from the surgical biopsy. This is especially true in young patients or those with limited lung reserve.
Treatment of pulmonary salivary type tumors is surgical. As most of these tumors are centrally located, the risk of pneumonectomy is very high, especially with the wrong diagnosis of a more aggressive tumor. Therefore, tumors require high clinical suspicion and a center experienced with parenchymal preserving techniques such as sleeve resections. Radical procedures like pneumonectomy are unnecessary.7
Complete resection of the tumor provides the only curative therapy.1 In high-grade tumors and those with incomplete resection, adjuvant therapies with chemotherapy or radiotherapy have been added. However, the disease-free and overall survival are low.1 Prognosis depends on the histological grade, malignant tumors staging, especially lymph node metastasis and completion of resection.2 Those with stage I and II disease are reported to have 100% survival, whereas those with stage 3 and 4, survival may be as poor as 43.6% in five years (p < 0.001).1 The overall risk of recurrence is 22.6%. Recurrence was only seen in high-grade and incompletely resected tumors. The incomplete resection recurrence rate is 37.5%.8 These results emphasize close follow-up in those with high-grade tumors or those with incomplete resection.
Conclusion
MEC is a rare salivary gland type tumor in the respiratory tract. It is important to distinguish it from high-grade aggressive tumors to avert unnecessary resection. However, confirmative differentiation from adenocarcinoma may be difficult from preoperative biopsy. Thus, a high-degree of clinical suspicion and awareness of histological limitations are necessary for its correct management.
Disclosure
The authors declared no conflicts of interest. Written consent was obtained from the patient.
references
- 1. Song Z, Liu Z, Wang J, Zhu H, Zhang Y. Primary tracheobronchial mucoepidermoid carcinoma–a retrospective study of 32 patients. World J Surg Oncol 2013 Mar;11:62.
- 2. Yousem SA, Hochholzer L. Mucoepidermoid tumors of the lung. Cancer 1987 Sep;60(6):1346-1352.
- 3. Falk N, Weissferdt A, Kalhor N, Moran CA. Primary pulmonary salivary gland-type tumors: a review and update. Adv Anat Pathol 2016 Jan;23(1):13-23.
- 4. Wilkins EW Jr, Darling RC, Soutter L, Sniffen RC. A continuing clinical survey of adenomas of the trachea and bronchus in a general hospital. J Thorac Cardiovasc Surg 1963 Sep;46:279-291.
- 5. Ozlu C, Christopherson WM, Allen JD Jr. Mucoepidermoid tumors of the bronchus. J Thorac Cardiovasc Surg 1961 Jul;42:24-31.
- 6. Molina JR, Aubry MC, Lewis JE, Wampfler JA, Williams BA, Midthun DE, et al. Primary salivary gland-type lung cancer: spectrum of clinical presentation, histopathologic and prognostic factors. Cancer 2007 Nov;110(10):2253-2259.
- 7. Breyer RH, Dainauskas JR, Jensik RJ, Faber LP. Mucoepidermoid carcinoma of the trachea and bronchus: the case for conservative resection. Ann Thorac Surg 1980 Mar;29(3):197-204.
- 8. Kang DY, Yoon YS, Kim HK, Choi YS, Kim K, Shim YM, et al. Primary salivary gland-type lung cancer: surgical outcomes. Lung Cancer 2011 May;72(2):250-254.