Platelet transfusion is an essential part of treating hematological malignancies, marrow failure, and hematopoietic stem cell transplantation.1,2 Platelet transfusion refractoriness (PTR) can be defined as failure to achieve a satisfactory platelet count in a patient after two or more consecutive transfusions of allogeneic platelets.3,4 It is associated with a number of adverse outcomes including longer hospital stays,5 increased risk of bleeding,6,7 decreased survival,7 and higher inpatient hospital costs.3,5 The current incidence of PTR ranges from 5% to 14% in hematological patients.8–11 The problem is greater in patients with multiple transfusions as 30%–70% become refractory to random donor platelet (RDP) transfusions.12–14
PTR causes are multifactorial, with 80% attributed to non-immunological causes and 20% to immunological causes.15–17 The latter is often attributed to antibodies to human leukocyte antigen (HLA) and/or human platelet antigens (HPAs). Several approaches have been developed to address the problem of immune-mediated platelet refractoriness. One of the most frequently used methods is HLA matching, which is highly effective and represents the routine approach to the management of refractory patients in a number of institutions.18,19 HLA matching requires the availability of large numbers of HLA-typed donors.2 Even large blood suppliers periodically have difficulty identifying HLA-matched donors for some patients.20 In addition, HLA typing techniques are time-consuming and costly. Also, it has been reported that about 40%–50% of HLA-matched platelet transfusion events do not result in adequate increments.21
Platelet crossmatching assays are a relatively low-cost and rapid alternative to the HLA-matched approach for the management of platelet refractoriness.22–24 Crossmatching assays have been used for the identification of candidate platelet donors and may be beneficial for patients in whom refractoriness is due to HPA alloimmunization.25
Despite the routine use of platelet crossmatching at many institutions, it is still not implemented as a tool for the management of refractory patients in Egyptian institutions.
Here, we present transfusion-related outcomes observed at Alexandria’s main university hospitals to determine whether platelet crossmatching can effectively identify platelet units that will improve the post-transfusion platelet counts.
Moreover, We sought to evaluate the role of platelet crossmatching assay in the management of patients with hematological disorders refractory to platelet transfusion and the effect of HLA-mediated platelet refractoriness.
Methods
This prospective study was conducted on 40 patients with different hematological disorders (24 males and 16 females), of which 28 were adults and 12 were pediatrics. Their age ranged from 6 to 73 years, with a median age of 34.0 years. They were identified as refractory after receiving RDP transfusions. All were presented to the hematology unit of Alexandria’s main university hospitals between May 2020 and March 2021. They received 60 ABO-compatible platelet transfusions (ranging from one to four transfusions per patient). Platelets were stored at 20–24 °C with continuous agitation for a maximum of three days. Patients with evidence of non-immunological causes of platelet refractoriness were excluded. This study received approval from the Medical Ethics Committee of the Faculty of Medicine, Alexandria University, Egypt. Written informed consent was obtained from each patient/guardian participating in the study. Platelet cross-matching was performed for all patients selected to be refractory to random platelet transfusion based on their 24-hour post-transfusion corrected count increment (CCI) of < 2500/µL after at least two consecutive transfusions. The CCI was calculated using the following formula:26
CCI = [post-transfusion platelet count (109/L) - pre-transfusion platelet count (109/L)] × [body surface area (m2)]/ [platelet dose transfused (1011)].
After performing platelet crossmatching, a complete blood picture was done at one hour and 24 hours after platelet transfusion. CCI was calculated. Other formulae to calculate platelet increment (PI) and percentage platelet recovery (PPR) were also calculated.20,27 Pre- and post-transfusion platelet counts were estimated on Advia 2120i hematology analyzer (Siemens, Germany), and patients’ prior transfusion history was accessed from hospital records.
Platelet cross-match assays were performed using the solid-phase red cell adherence (SPRCA) technique with Capture-P Ready Screening (Immucor, Norcross, GA, USA) on the automated apparatus (NEO; Immucor 4th generation) for the detection of IgG antibodies to platelet specific antigens. Briefly, the serum is incubated in platelet-coated wells to allow antibodies, if present, to bind to the platelets. Unbound immunoglobulins (Igs) are then washed from the wells and replaced with a suspension of anti-IgG-coated indicator red cells. Centrifugation brings the indicator red cells in contact with antibodies bound to the immobilized platelets. The negative test shows a button of indicator red cells at the bottom of the test well with no readily detectable area of adherence and is considered compatible, while the positive test shows adherence of indicator red cells to part of or the entire reaction surface and is considered incompatible.
Patients' serum samples were collected at
-80 oC for HLA antibody detection using the enzyme-linked immunosorbent assay (ELISA) technique (Glory Science Co., Ltd, Del Rio, TX, USA). ELISA was performed according to the manufacturer’s instruction using Bio Rrad PW40 Microplate Washer and PR 4100 microplate reader.
Data were analyzed using SPSS Statistics (IBM Corp. Released 2011. IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp.). Categorical data were represented as numbers and percentages. We used the chi-square test to investigate the association between the categorical variables. Alternatively, Fisher’s exact correction test was applied when the expected cell counts were less than five. We used odds ratio (OR) to calculate the ratio of the odds and 95% CI of an event occurring in one risk group to the odds of it happening in the non-risk group. In addition, sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy for agreement was used. The significance of the obtained results was judged at the 5% level.

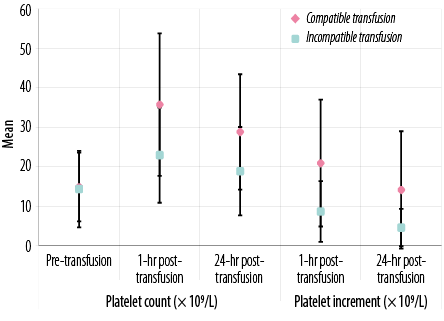 Figure 1: Laboratory data of all transfusion events in patients under study according to platelet count (× 109/L) and platelet increment.
Figure 1: Laboratory data of all transfusion events in patients under study according to platelet count (× 109/L) and platelet increment.

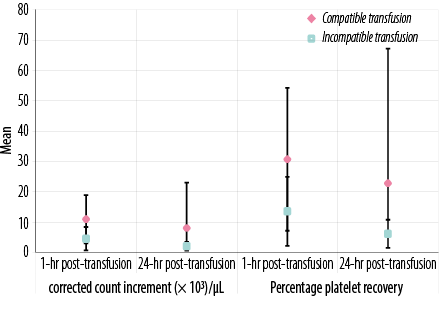 Figure 2: Laboratory data of all transfusion events in patients under study according to corrected count increment (× 103/µL) and percentage platelet recovery.
Figure 2: Laboratory data of all transfusion events in patients under study according to corrected count increment (× 103/µL) and percentage platelet recovery.
Results
Sixty percent (24/40) of refractory patients were males, and 40.0% (16/40) were females. Their age ranged from 6–73 years, with a median age of 34.0 years. Seventy percent of the studied patients had aplastic anemia, 20.0% had acute myeloid leukemia, and 10.0% had acute lymphoblastic leukemia.
There were significant differences between patients who received cross-matched compatible and those who received cross-matched incompatible platelets in one hour and 24 hours post-transfusion platelet counts, post-transfusion PI, CCI, and PPR (p < 0.05 for all) [Figures 1 and 2 and supplementary Table 1].
Table 1: Comparison between cross-matched compatible and incompatible transfusions at one hour and 24 hours post-transfusion.
|
CCI (× 103)
|
|
|
|
|
1-hour post-transfusion
|
n = 47
|
n = 13
|
|
|
Good response (> 5)
|
30 (63.8)
|
3 (23.1)
|
0.009*
|
|
Poor response (< 5)
|
17 (36.2)
|
10 (76.9)
|
|
|
24-hour post-transfusion
|
n = 47
|
n = 13
|
|
|
Good response (> 2.5)
|
44 (93.6)
|
3 (23.1)
|
< 0.001FE*
|
|
Poor response (< 2.5)
|
3 (6.4)
|
10 (76.9)
|
|
|
CCI (× 103)
|
|
|
|
|
1-hour post-transfusion
|
n = 35
|
n = 10
|
|
|
Good response (> 5)
|
22 (62.9)
|
2 (20.0)
|
0.029FE*
|
|
Poor response (< 5)
|
13 (37.1)
|
8 (80.0)
|
|
|
24-hour post-transfusion
|
n = 35
|
n = 10
|
|
|
Good response (> 2.5)
|
33 (94.3)
|
2 (20.0)
|
< 0.001FE*
|
|
Poor response (< 2.5)
|
2 (5.7)
|
8 (80.0)
|
|
|
CCI (× 103)
|
|
|
|
|
1-hour post-transfusion
|
n = 12
|
n = 3
|
|
|
Good response (> 5)
|
8 (66.7)
|
1 (33.3)
|
0.525FE
|
|
Poor response (< 5)
|
4 (33.3)
|
2 (66.7)
|
|
|
24-hour post-transfusion
|
n = 12
|
n = 3
|
|
|
Good response (> 2.5)
|
11 (91.7)
|
1 (33.3)
|
0.081FE
|
CCI: corrected count increment; FE: Fisher’s exact. *Statistically significant at p ≤ 0.05.
Table 1 shows platelet transfusion response after crossmatching. Compatible transfusions showed a better response than incompatible transfusions at both one hour and 24 hours for all studied patients (total of adults and pediatrics). Pediatric results are not significant either at one hour or 24 hours (p = 0.525, p = 0.081, respectively).
Also, patients who received cross-match incompatible platelets showed higher risk to develop poor response either at one hour (OR = 5.882, 95% CI: 1.421–24.355) or 24-hour post-transfusion (OR = 48.889, CI: 8.569–278.920) as shown in Table 2.
Table 2: Role of platelet crossmatching as a predictor of platelet refractoriness.
|
1-hour post-transfusion
|
|
|
|
|
|
Good response (> 5)
|
30 (63.8%)
|
3 (23.1%)
|
0.015*
|
1.000
|
|
Poor response (< 5)
|
17 (36.2%)
|
10 (76.9%)
|
5.882 (1.421–24.355)
|
|
24-hour post-transfusion
|
|
|
|
|
|
Good response (> 2.5)
|
44 (93.6%)
|
3 (23.1%)
|
1.000
|
CCI: corrected count increment; OR: odds ratio. *Statistically significant at p ≤ 0.05.
Moreover, patients with HLA alloimmunization showed higher risk to develop poor response either at one hour (OR = 7.442, 95% CI: 2.356–23.376) or 24 hours post-transfusion (OR = 5.882, CI: 5.882–24.355) [Table 3].
Table 3: Human leukocyte antigen (HLA) alloimmunization as predictor of platelet refractoriness.
|
1-hour post-transfusion
|
|
|
|
|
|
Good response (> 5)
|
8 (29.6)
|
25 (75.8)
|
0.001*
|
1.000
|
|
Poor response (< 5)
|
19 (70.4)
|
8 (24.2)
|
7.422 (2.356–23.376)
|
|
24-hour post-transfusion
|
|
|
|
|
|
Good response (> 2.5)
|
17 (63.0)
|
30 (90.9)
|
1.000
|
CCI: corrected count increment; OR: odds ratio.*Statistically significant at p ≤ 0.05.
Regarding platelet cross-matching, adequate CCI for compatible units was higher than incompatible units for both RDP and single donor platelet at one hour or 24 hours [Table 4]. Characteristics of transfusion events are shown in supplementary Table 2.
Table 4: Crossmatching as a predictor to response to platelet transfusion regarding the type of platelet donation at one hour and 24 hours post-transfusion.
|
1-hour post-transfusion CCI, × 103
|
|
Cross-match, SDP type
|
|
|
|
|
|
|
|
|
Compatible (-ve)
|
15 (93.8)
|
5 (71.4)
|
28.6
|
93.6
|
66.7
|
75.0
|
73.9
|
|
Incompatible (+ve)
|
1 (6.3)
|
2 (28.6)
|
|
Cross-match, RDP type
|
|
|
|
|
|
|
|
|
Compatible (-ve)
|
15 (88.2)
|
12 (60.0)
|
40.0
|
88.2
|
80.0
|
55.6
|
62.2
|
|
Incompatible (+ve)
|
2 (11.8)
|
8 (40.0)
|
|
24-hour post-transfusion CCI, × 103
|
|
Cross-match, SDP type
|
|
|
|
|
|
|
|
|
Compatible (-ve)
|
19 (95.0)
|
1 (33.3)
|
66.7
|
95.0
|
66.7
|
95.0
|
91.3
|
|
Incompatible (+ve)
|
1 (5.0)
|
2 (66.7)
|
|
Cross-match, RDP type
|
|
|
|
|
|
|
|
|
Compatible (-ve)
|
25 (92.6)
|
2 (20.0)
|
CCI: corrected count increment; SDP: single donor platelet; RDP: random donor platelet; Sen.: sensitivity; Spe.: specificity; PPV: positive predictive value; NPV: negative predictive value; Acc.: accuracy.
Discussion
Platelet transfusion therapy is lifesaving for patients with hematological disorders, but platelet refractoriness always poses a challenge due to alloimmunization to HLA and HPAs. A commonly used alternative to HLA-matched platelets is the transfusion of cross-match compatible platelets.23,28 There are surprisingly few reports describing the benefit obtained from using SPRCA assays to identify cross-matched compatible platelets.1,13,22,29
However, there is a paucity of Egyptian literature on platelet crossmatching and platelet refractoriness with RDP transfusion for patients with hematological disorders.
Our study revealed that mean post-transfusion count and CCI observed with the compatible platelet products were significantly higher than those observed in the same patients given randomly selected platelets before crossmatching assay. Additionally, patients who received compatible platelets showed better post-transfusion platelet count and CCI than incompatible transfusions at one and 24 hours.
The mean CCI of 10.96 × 103 achieved at 1-hour with compatible platelets in our study corresponds to a mean post-transfusion platelet count of 35.7 × 109/L, which is sufficient to avoid spontaneous bleeding. This CCI response to cross-matched units was significantly higher than that to comparable random platelet units for these patients, demonstrating benefits from crossmatch compatibility. The response to compatible platelets seen in our study is also consistent with that in prior studies that demonstrated a significant improvement in CCI using the SPRCA method to crossmatch platelets.22,29–33
Sayed et al,33 assessed the predictive value of a flow cytometric platelet crossmatching in 39 patients with acute leukemia (26 adults and 13 children). The transfusion response was better in children than in adults (p = 0.041). This is in contrast to our findings, which showed a better response in adults than children at both one hour and 24 hours post-transfusion (p = 0.029 and p < 0.001, respectively). Pediatric results were not significant either at one hour or 24 hours (p = 0.525 and p = 0.081, respectively). This may be attributed to differences in method sensitivity or the small number of pediatric patients in our study and needs to be studied in a larger group.
Platelet transfusion response was evaluated using the CCI, which was calculated at one hour and 24 hours post-transfusion. The cut-off values used were 5 × 103/µL at one hour and 2.5 × 103/µL at 24 hours in accordance with other studies.23,31,33,34 However, many studies used 7500/µL at one hour and 5000/µL at 24 hours as cut-offs.35–39 The lower cut-off values were used in this study due to endemic bilharzia and hepatitis C virus infection in the Egyptian people.
Platelet crossmatching was found to be a good predictor of transfusion response. A good response was reported in 63.8% of compatible transfusions, which was significantly higher than incompatible platelets (23.1%) at one hour or 24 hours (p = 0.015 and p < 0.001, respectively).
Our results were consistent with Rebulla et al,22 who used SPRCA automated technique and reported good response in 68% of evaluable transfusions.Sayed et al,33 reported a good response in 57.7% of compatible transfusion events, which may be due to the use of flow cytometric platelet crossmatching, a more sensitive method for crossmatching.
Anti-HLA antibodies were present in 45.0% of our patients. Kiefel et al,40 analyzed the sera of all patients using two techniques, monoclonal antibody immobilization of platelet antigens and complement-dependent lymphocytotoxicity (CDC), and observed anti-HLA antibodies in 42.9% of hemato-oncology patients. Moreover, Laundy et al,41 reported that 45%–70% of chronically transfused patients developed antibodies to HLA class I antigens using flow cytometry and CDC assay.
A multi-centric trial to reduce alloimmunization to platelets study found that the incidence of HLA alloimmunization was 3%–4% and 13%–14% in chronic recipients of leukoreduced and non-leukoreduced platelets, respectively.34,42 The high percentage of alloimmunization in our studied patients could be explained by the frequent use of RDP concentrates in our institution.
In our study, 11 females had a history of conception among the 16 females under the study. In addition, anti-HLA antibodies were present in seven females with six of those females having multiple pregnancies.
In agreement with previous studies, we found that platelet cross-matching was the best predictor for transfusion response, followed by HLA alloimmunization using multivariate analysis.31,33
Finally, platelet crossmatching using SPRCA assay showed higher sensitivity with RDP concentrates than SDP concentrates. Regarding RDP type of platelet transfusions, the assay showed 80.0% sensitivity, 92.6% specificity, 80.0% PPV, and 92.6% NPV at 24 hours post-transfusion. While for SDP type, crossmatching assay showed 66.7% sensitivity, 95.0% specificity, 66.7% PPV, and 95.0% NPV.
This was similar to the study conducted by Elhence et al,39 on 31 refractory patients using the modified antigen capture enzyme technique for platelet crossmatching. Their study showed high clinical sensitivity of 88% and NPV of 93.2%. The clinical sensitivity of 80% and NPV of 92.6% for RDP concentrates in the current study suggest that the test may be a valuable tool for better selection of RDP units, as the high NPV demonstrates a greater chance of an adequate response with cross-matched compatible platelets, and also to improve the outcome of response in refractory patients.
We recommend that for patients who need frequent platelet support, if SDP transfusions are not available, it is better to provide the patients with compatible units of RDP concentrates after cross-matching to reduce the risk of alloimmunization and improve the outcome of the response in refractory patients.
Conclusion
Platelet crossmatching using a commercially available SPRCA technique and HLA screening are effective, useful, and are rapid tools for better management of patients’ refractory to platelet transfusions.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
Acknowledgments
The authors thank all patients who have been involved in this study, the hematology and blood bank team members, and colleagues who assisted in this research.
references
- 1. Mangwana S, Simon N. Evaluation of the platelet crossmatching in oncology patients. Global J Transfu Med 2016;1(1):16.
- 2. Stroncek DF, Rebulla P. Platelet transfusions. Lancet 2007 Aug;370(9585):427-438.
- 3. Fagundes IS, Franz JM, Jobim MS, Arend A, Merzoni J, Cardone JM, et al. Diagnosis and treatment of immunological platelet refractoriness by histocompatibility. Hum Immunol 2020 May;81(5):197-201.
- 4. Wool GD, Brown N. Alloantibodies and Platelets. In: Maitta RW, editor. Immunologic concepts in transfusion medicine. London, Elsevier; 2020. p. 117-148.
- 5. Meehan KR, Matias CO, Rathore SS, Sandler SG, Kallich J, LaBrecque J, et al. Platelet transfusions: utilization and associated costs in a tertiary care hospital. Am J Hematol 2000 Aug;64(4):251-256.
- 6. Toor AA, Choo SY, Little JA. Bleeding risk and platelet transfusion refractoriness in patients with acute myelogenous leukemia who undergo autologous stem cell transplantation. Bone Marrow Transplant 2000 Aug;26(3):315-320.
- 7. Kerkhoffs JL, Eikenboom JC, van de Watering LM, van Wordragen-Vlaswinkel RJ, Wijermans PW, Brand A. The clinical impact of platelet refractoriness: correlation with bleeding and survival. Transfusion 2008 Sep;48(9):1959-1965.
- 8. Bub CB, Gonçalez AC, Barjas-Castro ML, Castro V. Prospective evaluation of platelet refractoriness in haematological patients in a single Brazilian institution. ISBT Sci Ser 2021;16(1):2-11.
- 9. Hu X, Cai H, Zheng L, Luo Y, Zhou J, Hui Y, et al. Clinical and immunological features of platelet transfusion refractoriness in young patients with de novo acute myeloid leukemia. Cancer Med 2020 Jul;9(14):4941-4948.
- 10. Hess JR, Trachtenberg FL, Assmann SF, Triulzi DJ, Kaufman RM, Strauss RG, et al. Clinical and laboratory correlates of platelet alloimmunization and refractoriness in the PLADO trial. Vox Sang 2016 Oct;111(3):281-291.
- 11. Comont T, Tavitian S, Bardiaux L, Fort M, Debiol B, Morère D, et al. Platelet transfusion refractoriness in patients with acute myeloid leukemia treated by intensive chemotherapy. Leuk Res 2017 Oct;61:62-67.
- 12. Philip J, Kumar S, Chatterjee T, Mallhi RS. Prevalence of alloimmunization to human platelet antigen glycoproteins and human leucocyte antigen class I in β thalassemia major patients in Western India. Indian J Hematol Blood Transfus 2014 Dec;30(4):309-312.
- 13. Kingsley S, Chacko MP, Amal P, Rebekah G, Leni GM, Dolly D. Frequency of platelet crossmatch positivity and predictive value for poor platelet increment among paediatric oncohaematology patients in India. Indian J Hematol Blood Transfus 2020 Jan;36(1):164-170.
- 14. Abraham AS, Chacko MP, Fouzia NA, Srivastava A, Daniel D. Antibodies to human platelet antigens form a significant proportion of platelet antibodies detected in Indian patients with refractoriness to platelet transfusions. Transfus Med 2018 Oct;28(5):392-397.
- 15. Ferreira AA, Zulli R, Soares S, Castro Vd, Moraes-Souza H. Identification of platelet refractoriness in oncohematologic patients. Clinics (Sao Paulo) 2011;66(1):35-40.
- 16. Solves Alcaina P. Platelet transfusion: and update on challenges and outcomes. J Blood Med 2020 Jan;11:19-26.
- 17. Zhou Z, Gao Y, Li X, Ren J, Liu Y, Li J. The clinical characteristics of patients with acute leukemia or stem cell transplantation exhibiting immune based platelet refractoriness. Transfus Apher Sci 2020 Jun;59(3):102725.
- 18. Petz LD, Garratty G, Calhoun L, Clark BD, Terasaki PI, Gresens C, et al. Selecting donors of platelets for refractory patients on the basis of HLA antibody specificity. Transfusion 2000 Dec;40(12):1446-1456.
- 19. Kreuger AL, Mäkelburg AB, Somers JA, Tomson B, van de Watering LM, van der Bom JG, et al. HLA-matched platelet transfusions are effective only in refractory patients with positive HLA antibody screening. Transfusion 2019 Nov;59(11):3303-3307.
- 20. Simon TL, McCullough J, Snyder EL, Solheim BG, Strauss RG. Rossi’s principles of transfusion medicine. USA, John Wiley & Sons; 2016.
- 21. Schiffer CA, Bohlke K, Delaney M, Hume H, Magdalinski AJ, McCullough JJ, et al. Platelet transfusion for patients with cancer: American society of clinical oncology clinical practice guideline update. J Clin Oncol 2018 Jan;36(3):283-299.
- 22. Rebulla P, Morelati F, Revelli N, Villa MA, Paccapelo C, Nocco A, et al. Outcomes of an automated procedure for the selection of effective platelets for patients refractory to random donors based on crossmatching locally available platelet products. Br J Haematol 2004 Apr;125(1):83-89.
- 23. Rebulla P. A mini-review on platelet refractoriness. Haematologica 2005 Feb;90(2):247-253.
- 24. Rajadhyaksha BS, Desai DP, Navkudkar AA. Platelet refractoriness. Glob J Transfus Med 2019;4(2):140.
- 25. Eisenberg S. Refractory response to platelet transfusion therapy. J Infus Nurs 2010 Mar-Apr;33(2):89-97.
- 26. Cohn CS. Platelet transfusion refractoriness: how do I diagnose and manage? Hematology Am Soc Hematol Educ Program 2020 Dec;2020(1):527-532.
- 27. Hod E, Schwartz J. Platelet transfusion refractoriness. Br J Haematol 2008 Jul;142(3):348-360.
- 28. Schmidt AE, Refaai MA, Coppage M. HLA-mediated platelet refractoriness. Am J Clin Pathol 2019 Mar;151(4):353-363.
- 29. Mangwana S, Kacker A, Simon N. Platelet compatibility and platelet antibodies detection: a step towards resolving dilemma in management of platelet refractoriness in oncology patients. Glob J Transfus Med 2019;4(2):148.
- 30. Wiita AP, Nambiar A. Longitudinal management with crossmatch-compatible platelets for refractory patients: alloimmunization, response to transfusion, and clinical outcomes (CME). Transfusion 2012 Oct;52(10):2146-2154.
- 31. Salama OS, Aladl DA, El Ghannam DM, Elderiny WE. Evaluation of platelet crossmatching in the management of patients refractory to platelet transfusions. Blood Transfus 2014 Apr;12(2):187-194.
- 32. Desai P, Sontakke P, Rajadhyaksha S, Navkudkar A. Correlation of platelet crossmatch results by solid phase red cell adherence assay (SPRCA) with post-transfusion platelet count increment in adult hemato-oncology patients of a tertiary care oncology centre in India. Transfus Apher Sci 2020 Oct;59(5):102842.
- 33. Sayed D, Bakry R, El-Sharkawy N, Zahran A, Khalaf MR. Flow cytometric platelet cross-matching to predict platelet transfusion in acute leukemia. J Clin Apher 2011;26(1):23-28.
- 34. McFarland J, Menitove J, Kagen L, Braine H, Kickler T, Ness P, et al; Trial to Reduce Alloimmunization to Platelets Study Group. Leukocyte reduction and ultraviolet B irradiation of platelets to prevent alloimmunization and refractoriness to platelet transfusions. N Engl J Med 1997 Dec;337(26):1861-1869.
- 35. Davis KB, Slichter SJ, Corash L. Corrected count increment and percent platelet recovery as measures of posttransfusion platelet response: problems and a solution. Transfusion 1999 Jun;39(6):586-592.
- 36. Schiffer CA, Anderson KC, Bennett CL, Bernstein S, Elting LS, Goldsmith M, et al; American Society of Clinical Oncology. Platelet transfusion for patients with cancer: clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol 2001 Mar;19(5):1519-1538.
- 37. Sacher RA, Kickler TS, Schiffer CA, Sherman LA, Bracey AW, Shulman IA; College of American Pathologists.Transfusion Medicine Resource Committee. Management of patients refractory to platelet transfusion. Arch Pathol Lab Med 2003 Apr;127(4):409-414.
- 38. Hod E, Schwartz J. Platelet transfusion refractoriness. Br J Haematol 2008 Jul;142(3):348-360.
- 39. Elhence P, Chaudhary RK, Nityanand S. Cross-match-compatible platelets improve corrected count increments in patients who are refractory to randomly selected platelets. Blood Transfus 2014 Apr;12(2):180-186.
- 40. Kiefel V, König C, Kroll H, Santoso S. Platelet alloantibodies in transfused patients. Transfusion 2001 Jun;41(6):766-770.
- 41. Laundy GJ, Bradley BA, Rees BM, Younie M, Hows JM. Incidence and specificity of HLA antibodies in multitransfused patients with acquired aplastic anemia. Transfusion 2004 Jun;44(6):814-825.
- 42. Seftel MD, Growe GH, Petraszko T, Benny WB, Le A, Lee C-Y, et al. Universal prestorage leukoreduction in Canada decreases platelet alloimmunization and refractoriness. Blood 2004 Jan;103(1):333-339. Blood 2004 Jan;103(1):333-339.
Supplementary tables
Table 1: Comparison between cross-matched compatible and incompatible transfusions according to laboratory data of all transfusion events.
|
Platelet count, 109/L
|
|
|
|
|
|
Pre-transfusion
|
14.8 ± 8.7
|
14.3 ± 9.7
|
283.00
|
0.686
|
|
1-hr post-transfusion
|
35.7 ± 18.0
|
22.9 ± 12.0
|
174.50*
|
0.019*
|
|
24-hr post-transfusion
|
28.7 ± 14.
|
18.8 ± 11.2
|
174.50*
|
0.019*
|
|
Platelet increment
|
|
|
|
|
|
1-hr post-transfusion
|
20. ± 16.0
|
8.6 ± 7.7
|
124.50*
|
0.001*
|
|
24-hr post-transfusion
|
14. ± 14.8
|
4.5 ± 4.7
|
98.0*
|
< 0.001*
|
|
CCI, 103/µL
|
|
|
|
|
|
1-hr post-transfusion
|
10.9 ± 7.9
|
4.5 ± 3.8
|
116.00*
|
0.001*
|
|
24-hr post-transfusion
|
8.0 ± 14.9
|
2. ± 1.4
|
55.00*
|
< 0.001*
|
|
Percentage platelet recovery
|
|
|
|
|
|
1-hr post-transfusion
|
30.7 ± 23.5
|
13.5 ± 11.3
|
132.50*
|
0.002*
|
U: Mann Whitney test; CCI: corrected count increment. *Statistically significant at p ≤ 0.05.
Table 2: Comparison between cross-matched compatible and incompatible transfusions according to characteristics of transfusion events.
|
Type of unit, n(%)
|
|
|
|
|
SDP
|
23 (38.3)
|
20 (42.6)
|
3 (23.1)
|
|
RDP
|
37 (61.7)
|
27 (57.4)
|
10 (76.9)
|
|
Platelet dose transfused, × 1011
|
|
|
|
|
Mean ± SD
|
3.3 ± 1.7
|
3.3 ± 1.7
|
3.4 ± 1.6
|
|
Median (min–max)
|
3 (1.5–10.0)
|
3 (1.5–10.0)
|
3 (1.5–6.0)
|
|
No of platelet units
|
|
|
|
|
Mean ± SD
|
6.6 ± 3.3
|
6.5 ± 3.4
|
6.8 ± 3.2
|
|
Median (min–max)
|
6 (3–20)
|
6 (3–20)
|
6 (3–12)
|
|
Storage time units (days), n(%)
|
|
|
|
|
1
|
30 (50.0)
|
24 (51.1)
|
6 (46.2)
|
|
2
|
23 (38.3)
|
17 (36.2)
|
6 (46.2)
|
|
3
|
7 (11.7)
|
6 (12.8)
|
1 (7.7)
|
|
Blood group, n(%)
|
|
|
|
|
O
|
16 (26.7)
|
14 (29.8)
|
2 (15.4)
|
|
A
|
19 (31.7)
|
13 (27.7)
|
6 (46.2)
|
|
B
|
16 (26.7)
|
12 (25.5)
|
4 (30.8)
|
SDP: single donor platelet; RDP: random donor platelet.