In 2015, the World Health Organization estimated that 3.5% of the world’s population had chronic hepatitis B (CHB) virus infection, corresponding to 257 million people. A report of deaths related to CHB complications in 2015 indicated 887 000 deaths worldwide.1 Most deaths are related to decompensated liver cirrhosis and the development of hepatocellular carcinoma (HCC).1 The hepatitis B virus (HBV) vaccine introduction has decreased the incidence of hepatitis B viral infection.2–6 The risk of CHB infection is high among patients infected during the early years of life.7
The estimated prevalence of CHB in Oman before introducing HBV vaccination was 2–7%.8 Hepatitis B vaccine was introduced in Oman in 1990. Catch-up vaccination of school students born before the introduction of HBV vaccination complemented the introduction program of HBV vaccination. A follow-up study in 2005 showed the HBV vaccine coverage rate to be > 95%.9 In addition to the vaccination program, there were other preventative measures introduced to reduce the risk of HBV transmission, such as blood donation screening and screening high-risk populations (e.g., healthcare workers, patients on regular blood transfusion, and hemodialysis patients).10
Despite the reported association between CHB and liver complications such as decompensated cirrhosis and development of HCC, there is no available data from Oman illustrating the CHB related liver cirrhosis that could support evaluating the burden of HBV and help planning preventative strategies and measures to reduce the burden of CHB outcome. This study was conducted to assess the prevalence of CHB related liver cirrhosis in Oman.
Methods
This cross-sectional study looked at patients diagnosed with liver cirrhosis at Sultan Qaboos University Hospital and Armed Forces Hospital between January 2006 and April 2013. These two hospitals are tertiary hospitals in the capital city of Oman, Muscat, where most of the cases from other regions are referred. Therefore, the sample in this study could be a close representation of CHB related liver cirrhosis in the country. Patients’ data were retrieved from the two computerized hospital information systems and patient interviews during hospital visits.
All pediatric and adult patients with liver cirrhosis were included. Patients with missing data and those with pre- and post-hepatic portal hypertension were excluded from this study.
Demographic data such as age, gender, medical history that includes comorbidities such as drug history, diabetes mellitus (DM), or other components of metabolic syndrome, heart failure, smoking and alcohol consumption, and family history of liver diseases were collected.
Blood investigations including complete blood count, coagulation profile, liver chemistry, electrolytes, renal function, hepatitis B and C serology, viral loads in positive cases, alpha 1 antitrypsin level, serum ceruloplasmin, iron profile and serum ferritin, and antinuclear antibody and autoimmune profile were collected. Results of abdominal ultrasound, computed tomography (CT) scan and/or magnetic resonance imaging, gastroscopy findings, as well as liver biopsy results were also collected.
The diagnosis of liver cirrhosis was based on laboratory and radiological investigations and liver histology was the gold standard test. Patients were categorized into three categories based on the degree of accuracy of the diagnosis of liver cirrhosis. The categories are definite cirrhosis, probable cirrhosis, and possible cirrhosis. A description of the criteria used to categorize the patients is shown in Table 1.
Statistical analysis was done using Epi infoTM version 7. This included calculating the frequencies of cirrhosis etiology, calculating the median values of age, and laboratory data.
Results
The total number of identified patients diagnosed with cirrhosis from both hospitals was 469. Fifty patients were excluded due to different reasons. The final analysis included 419 patients [Figure 1]. When classified based on the accuracy of cirrhosis diagnosis, 70.9%, 10.3%, 18.9% of the study cohort had definite, probable, and suspected liver cirrhosis, respectively [Table 1].

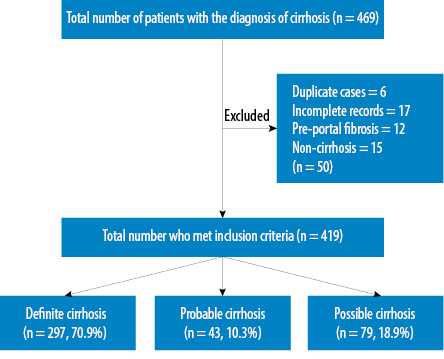 Figure 1: Flow chart showing the results of patient's selection process.
Figure 1: Flow chart showing the results of patient's selection process.
Table 1: Categories depending on the accuracy of cirrhosis diagnosis.
|
Definite cirrhosis |
- Either histological features of cirrhosis and/or radiological features suggestive of liver cirrhosis (irregular or undulated liver surface with heterogeneous or coarse echotexture) with splenomegaly, collateral vessels, ascites and dilated portal vein (PV).
- With/without biochemical tests showing raised aminotransferases (with aspartate aminotransferase > alanine aminotransferase usually), high bilirubin, and low albumin associated with low platelet count and high prothrombin time (PT) or international normalized ratio (INR).
|
|
Probable cirrhosis |
- Either irregular or undulated liver surface or heterogeneous or coarse echotexture on radiological images. With or without extrahepatic features such as enlarged spleen, collateral vessels, ascites, and dilated PV
- With/without biochemical tests showing high bilirubin, low albumin, low platelet count, or increased INR or PT.
|
Table 2: Patients’ demographics and clinical history.
|
General demographic profile |
|
Number of patients |
419 |
|
|
Age, years (median) |
59 |
|
|
Gender |
|
Male |
283 |
67.5 |
|
Female |
136 |
32.5 |
|
Ethnicity |
|
Omani |
407 |
97.1 |
|
Non-Omani |
12 |
2.9 |
|
Patient history |
|
Diabetes mellitus |
|
Yes |
200 |
47.7 |
|
No |
59 |
14.1 |
|
Not available |
160 |
38.2 |
|
Family history of liver disease |
|
Yes |
15 |
3.6 |
|
No |
31 |
7.4 |
|
Not available |
373 |
89 |
|
Alcohol consumption |
|
Yes |
93 |
22.2 |
|
No |
86 |
20.5 |
|
Not available |
240 |
57.3 |
|
Smoking |
|
Yes |
58 |
13.8 |
|
No |
84 |
20.0 |
Table 3: Hepatitis B virus (HBV) status of the study cohort.
|
Current and previous HBV infection |
215 (51.3) |
63 (29.3) |
HBsAg
Isolate anti-HBc |
|
Current HBV infection |
83 (38.6) |
16 (19.9) |
Positive HBsAg |
|
Previous HBV infection
(with or without HBV immunity) |
79 (36.7) |
26 (32.9) |
Isolate anti-HBc and/or
anti-HBv + anti-HBs |
|
HBV with HCV co-infection† |
53 (24.7) |
21 (39.6) |
anti-HBc + anti-HCV |
|
Hepatitis B activity |
|
Hepatitis B Viral Load |
47 (21.9) |
19 (40.4) |
HBVDNA |
anti-HBc: anti hepatitis B core antibody; anti-HBs: anti hepatitis B surface antibody.; The numbers and percentages in this table represent hepatitis B patients with cirrhosis (total 215) and not the entire cohort.; † 45 patients had past HBV with co-HCV infection.
The demographic characteristics are shown in Table 2. Two-thirds of the patients with cirrhosis were males. The median age of the study sample was 59 years [Figure 2]. The majority (97.1%) of patients with cirrhosis were Omanis. Almost half of the cohort had diabetes, and 22.2% indicated consumption of alcohol with no information available regarding the amount and duration of alcohol consumption. Figure 3 illustrates the etiologies of cirrhosis in the study sample.

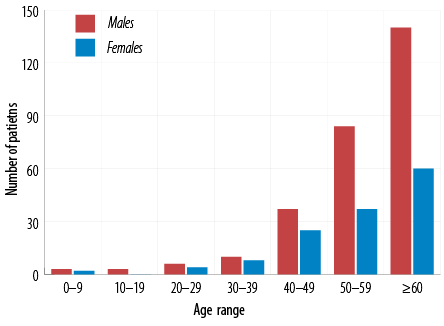 Figure 2: Age distribution of patients with cirrhosis.
Figure 2: Age distribution of patients with cirrhosis.
Most patients (69.3%) had viral hepatitis as etiology for liver cirrhosis. More than half of the study cohort (51.3%) had evidence of previous or current infection with HBV. Hepatitis B e Antigen (HBeAg) was found in 3.3% of CHB patients. HBV DNA was detected in 47 patients (21.9%), of which 20 patients had a high viral load > 2000IU/mL [Table 3].
Most patients with HBV related liver cirrhosis were males (70.7%) compared to females (29.3%). HBV mono-infection is more common than HCV mono-infection (38.6% vs. 18.0%), p < 0.010 [Figure 3]. Patients with hepatitis B related liver cirrhosis were younger than those with other etiologies. Hepatitis B coinfection with HCV was found in 24.7% of HBV patients with cirrhosis [Table 3].

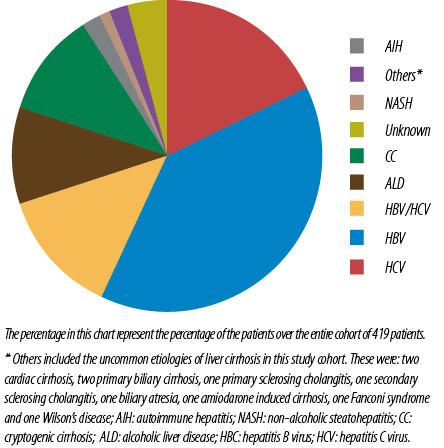 Figure 3: Etiology of cirrhosis, N = 419.
Figure 3: Etiology of cirrhosis, N = 419.
Data for HBV immunity was available for 328 patients (78.3%) [Figure 4]. Of these, 36.7% were positive for hepatitis B surface antibody (anti-HBs). Negative anti-HBs was found in 34.1% of the cohort with 29.9% having unknown immunity status. The complete hepatitis B serology was not available in 3.7% of patients with anti-HBs. The majority of those with positive anti-HBs (27.1% out of the 36.7%) were immune due to previous infection (positive anti-HBs and antibodies to hepatitis B core antigen (anti-HBc)] with 5.2% only due to vaccination (positive anti-HBs and negative anti-HBc).

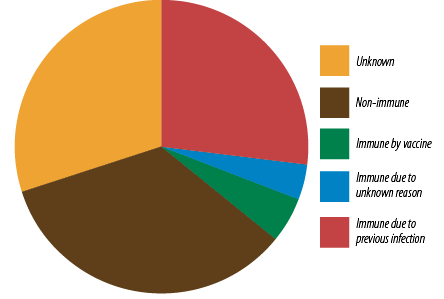 Figure 4: Hepatitis B virus immunity status
Figure 4: Hepatitis B virus immunity status
(n = 328).
Discussion
This study was conducted to investigate the association between CHB and liver cirrhosis in Oman. The majority of patients (97.1%) were Omani. This high percentage of Omani patients included in this study can reduce the effect of synergistic factors that accelerate the process of fibrosis among CHB patients, such as HBV genotypes and exposure to aflatoxins.11
HBV infection among patients with cirrhosis is correlated with the prevalence of the virus in that region. It is estimated that HBV accounts for 5% of liver cirrhosis in low prevalence areas and up to 57% in high prevalence areas such as East Asia.12 Based on that estimation, the contribution of HBV to liver cirrhosis in the Eastern Mediterranean region including Oman would be around 35%. If the same estimation is applied to the contribution of HBV to liver cirrhosis in this cohort, it would be equal to 19.6%. However, this estimated percentage is lower than what the current study indicates (38.6%). This discrepancy is likely due to multiple factors. Perz et al,12 based their estimation on studies from Saudi Arabia and Tunisia. The prevalence of HBV in Oman was considered intermediate before the introduction of HBV vaccination compared to Saudi Arabia and Tunisia, which were considered high and intermediate prevalence, respectively.13,14 In addition, the quoted studies used to estimate the prevalence in different countries differ markedly in the design, population, and methodology used.
There are certain factors that may accelerate the process of fibrosis among CHB patients and are present among our study patients. The probability of HBV clearance is higher among females due to unknown mechanisms.15 In addition, females tend to have a lower rate of accelerated fibrosis due to inhibition of activated stellate cells by hormones, mainly estrogen.16 This could explain why the majority of our patients in this study were males.
In countries with intermediate and high prevalence, the majority of patients acquire the infection in the early years of life.17 With a prolonged duration of HBV infection, the rate of fibrosis and hence cirrhosis is higher.18 The estimated prevalence of CHB in Oman before the introduction of HBV vaccination was 2–7%.19 This factor can explain why the median age of our patients was 59 years [Table 2].
DM and the associated non-alcoholic fatty liver disease increase the risk of developing advanced fibrosis.20 In a large prospective cohort study, DM doubled the risk of chronic non-alcoholic liver disease and HCC.21 This might explain one of the cirrhosis risk factors in this study population, as almost half of the cohort had DM [Table 2].
Acute and chronic alcohol consumption are reported to cause significant necroinflammatory changes within the liver parenchyma22 and alter cellular communication leading to cellular death.23 The risk of liver fibrosis progression in patients with CHB is therefore increased with the synergistic effect of alcohol consumption.24 The risk of cirrhosis among CHB patients who consume alcohol is also affected by other factors such as duration of alcohol intake, gender, and obesity.25 In the current study, alcohol consumption was reported by 22.2% of the patients. However, due to the retrospective design of our study, important information such as the amount and duration of alcohol consumption were not available. Therefore, the assessment of the synergetic effect of alcohol consumption on the CHB patients was not possible.
Fifty-one percent (51.3%) of our patients had isolated anti-HBc (HBsAg negative). This is higher than the reported prevalence of isolated anti-HBc among Omani blood donors of 20.5%.26 This difference could be related to the fact that blood donors in the previous study are younger than the patients in our cohort who were most likely HBV vaccinated and therefore the prevalence of isolated anti-HBc is likely to be low in their study.
The majority of our patients were HBeAg-negative chronic hepatitis B. This finding is similar to the reported studies from areas of intermediate to high prevalence regions.27 Once seroconversion from HBeAg to anti-HBe is achieved, suppression of HBV DNA is expected. Therefore, HBeAg-negative chronic hepatitis B patients tend to have low viral load and favorable prognosis with a low-risk of progression to liver cirrhosis or HCC.28 However, HBV of some of those HBeAg-negative CHB patients may develop a mutation in the pre-core or core promoter region, leading to significant viremia and progression to advanced fibrosis.29
We detected HBV DNA in 21.9% of patients with cirrhosis. A high viral load (more than 2000 IU/ml) was found in 42.6%. HBV DNA is the best measure of HBV replication. However, HBV DNA levels may fluctuate in patients who have HBeAg-negative chronic hepatitis B reaching normal or high normal at times. Previous studies have shown a reduction of HBV DNA with advanced liver fibrosis. Despite the reduction in HBV DNA, the liver inflammation continued leading to further fibrosis.30
Anti-HBs seroconversion is considered the target clinical endpoint in patients with chronic HBV infection.31 Anti-HBs was present in 36.7% of our patients. The majority (27.1% out of 36.7%) were due to previous infection. Once seroconversion to anti-HBs occurs, there should be no further liver inflammation or fibrosis. Therefore, we can postulate that cirrhosis occurred in this cohort before seroconversion unless other risk factors for progression toward cirrhosis such as alcohol consumption, co-infection with HCV or presence of non-alcoholic steatohepatitis exist in addition to chronic hepatitis B.
HBV co-infection with HCV accelerates liver fibrosis leading to early cirrhosis. The synergistic effect of HCV co-infection leading to advanced fibrosis has been mentioned in many studies.23–34 The effect of HBV and HCV co-infection have been described among isolated anti-HBc.35–37 The global estimation of HBV and HCV co-infection is around 10–15%.16 Our study showed that 12.6% of patients with liver cirrhosis had HBV and HCV co-infection.
HCV is usually the dominant virus in HBV co-infection with HCV.38 However, the persistence of low-level HBV replication within the hepatocytes induces chronic immune-related inflammation that increases the severity of liver disease and marked fibrosis.36,39
The study showed that only 5.2% of patients were vaccinated for HBV, and 34.1% had no immunity against HBV. This is most likely because most of those infected with hepatitis B were born before the introduction of vaccination in 1990.
The current study has multiple strengths. The sample size is relatively large with a good representation of the population given that the majority (97.1%) were Omanis representing different parts of the country. The classification of patients into three different groups using well-defined inclusion and exclusion criteria based on objective criteria adds to the strength of the study.
The major limitation of our study is its retrospective nature. Data related to certain cofactors that can attribute to liver cirrhosis, such as alcohol consumption, presence of metabolic syndrome, and other etiologies of liver cirrhosis was missing. Therefore, it was difficult to evaluate the synergistic effect of some cofactors and HBV in the etiology of cirrhosis in this study. Another limitation was the unavailability of complete HBV serological markers for some patients. This might underestimate the role of chronic HBV infection as a risk factor for liver cirrhosis.
Conclusion
Serological markers of chronic HBV infection are present in a large number of patients with liver cirrhosis in Oman. The majority of the patients are males of older age group born before the introduction of HBV vaccination. Screening of high- risk patients is essential to reduce the complications of HBV infection in Oman. Further research is essential to assess the role of contributing factors in the progression of CHB to cirrhosis in Oman.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
references
- 1. World Health Organization. Global hepatitis report 2017. Geneva: World Health Organization; 2017 [cited 2017 July 13]. Available from: https://www.who.int/publications/i/item/global-hepatitis-report-2017pdf.
- 2. Liu J, Liang W, Jing W, Liu M. Countdown to 2030: eliminating hepatitis B disease, China. Bull World Health Organ 2019 Mar;97(3):230-238.
- 3. Mandal S. Introduction of universal infant hepatitis B immunisation in the UK- paving the way to elimination. Hum Vaccin Immunother 2019;15(2):440-443.
- 4. Duffell EF, Hedrich D, Mardh O, Mozalevskis A. Towards elimination of hepatitis B and C in European Union and European Economic Area countries: monitoring the World Health Organization’s global health sector strategy core indicators and scaling up key interventions. Euro Surveill 2017 Mar;22(9):22.
- 5. Childs L, Roesel S, Tohme RA. Status and progress of hepatitis B control through vaccination in the South-East Asia Region, 1992-2015. Vaccine 2018 Jan;36(1):6-14.
- 6. Woodring J, Pastore R, Brink A, Ishikawa N, Takashima Y, Tohme RA. Progress toward hepatitis B control and elimination of mother-to-child transmission of hepatitis B virus - Western Pacific Region, 2005-2017. MMWR Morb Mortal Wkly Rep 2019 Mar;68(8):195-200.
- 7. Katamba PS, Mukunya D, Kwesiga D, Nankabirwa V. Prenatal hepatitis B screening and associated factors in a high prevalence district of Lira, northern Uganda: a community based cross sectional study. BMC Public Health 2019 Jul;19(1):1004.
- 8. Al Awaidy S, Abu-Elyazeed R, Al Hosani H, Al Mulla A, Al Busaiedy S, Al Amiry A, et al. Sero-epidemiology of hepatitis B infection in pregnant women in Oman, Qatar and the United Arab Emirates. J Infect 2006 Mar;52(3):202-206.
- 9. Al Awaidy ST, Bawikar SP, Al Busaidy SS, Al Mahrouqi S, Al Baqlani S, Al Obaidani I, et al. Progress toward elimination of hepatitis B virus transmission in Oman: impact of hepatitis B vaccination. Am J Trop Med Hyg 2013 Oct;89(4):811-815.
- 10. Joshi SR, Shah Al-Bulushi SN, Ashraf T. Development of blood transfusion service in Sultanate of Oman. Asian J Transfus Sci 2010 Jan;4(1):34-40.
- 11. Kramvis A. The clinical implications of hepatitis B virus genotypes and HBeAg in pediatrics. Rev Med Virol 2016 Jul;26(4):285-303.
- 12. Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol 2006 Oct;45(4):529-538.
- 13. Abdo AA, Sanai FM, Al-Faleh FZ. Epidemiology of viral hepatitis in Saudi Arabia: are we off the hook? Saudi J Gastroenterol 2012 Nov-Dec;18(6):349-357.
- 14. Ezzikouri S, Pineau P, Benjelloun S. Hepatitis B virus in the Maghreb region: from epidemiology to prospective research. Liver Int 2013 Jul;33(6):811-819.
- 15. Ruggieri A, Gagliardi MC, Anticoli S. Sex-dependent outcome of hepatitis B and C viruses infections: synergy of sex hormones and immune responses? Front Immunol 2018 Oct;9:2302.
- 16. Fattovich G, Bortolotti F, Donato F. Natural history of chronic hepatitis B: special emphasis on disease progression and prognostic factors. J Hepatol 2008 Feb;48(2):335-352.
- 17. Ghendon Y. Perinatal transmission of hepatitis B virus in high-incidence countries. J Virol Methods 1987 Aug;17(1-2):69-79.
- 18. Liaw YF. Natural history of chronic hepatitis B virus infection and long-term outcome under treatment. Liver Int 2009 Jan;29(Suppl 1):100-107.
- 19. Custer B, Sullivan SD, Hazlet TK, Iloeje U, Veenstra DL, Kowdley KV. Global epidemiology of hepatitis B virus. J Clin Gastroenterol 2004 Nov-Dec;38(10)(Suppl 3):S158-S168.
- 20. Targher G, Lonardo A, Byrne CD. Nonalcoholic fatty liver disease and chronic vascular complications of diabetes mellitus. Nat Rev Endocrinol 2018 Feb;14(2):99-114.
- 21. El-Serag HB, Tran T, Everhart JE. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology 2004 Feb;126(2):460-468.
- 22. Murata T, Takanari H, Watanabe S, Tanaka T, Suzuki S. Enhancement of chronic viral hepatitic changes by alcohol intake in patients with persistent HBs-antigenemia. Am J Clin Pathol 1990 Sep;94(3):270-273.
- 23. Dolganiuc A. Alcohol and viral hepatitis: role of lipid rafts. Alcohol Res 2015;37(2):299-309.
- 24. Stroffolini T, Cotticelli G, Medda E, Niosi M, Del Vecchio-Blanco C, Addolorato G, et al. Interaction of alcohol intake and cofactors on the risk of cirrhosis. Liver Int 2010 Jul;30(6):867-870.
- 25. Gramenzi A, Caputo F, Biselli M, Kuria F, Loggi E, Andreone P, et al. Review article: alcoholic liver disease–pathophysiological aspects and risk factors. Aliment Pharmacol Ther 2006 Oct;24(8):1151-1161.
- 26. Kaminski G, Alnaqdy A, Al-Belushi I, Nograles J, Al-Dhahry SH. Evidence of occult hepatitis B virus infection among Omani blood donors: a preliminary study. Med Princ Pract 2006;15(5):368-372.
- 27. Funk ML, Rosenberg DM, Lok AS. World-wide epidemiology of HBeAg-negative chronic hepatitis B and associated precore and core promoter variants. J Viral Hepat 2002 Jan;9(1):52-61.
- 28. Liaw Y-F, Chu C-M. Hepatitis B virus infection. Lancet 2009 Feb;373(9663):582-592.
- 29. Hadziyannis SJ, Vassilopoulos D. Hepatitis B e antigen-negative chronic hepatitis B. Hepatology 2001 Oct;34(4 Pt 1):617-624.
- 30. Kim JD, Choi JY, Bae SH, Yoon SK, Yang JM, Han NI, et al. Hepatitis B virus load in serum does not reflect histologic activity in patients with decompensated cirrhosis. Clin Gastroenterol Hepatol 2010 Jan;8(1):60-65.
- 31. Chevaliez S, Pawlotsky J-M. Diagnosis and management of chronic viral hepatitis: antigens, antibodies and viral genomes. Best Pract Res Clin Gastroenterol 2008;22(6):1031-1048.
- 32. Liu CJ, Liou JM, Chen DS, Chen PJ. Natural course and treatment of dual hepatitis B virus and hepatitis C virus infections. J Formos Med Assoc 2005 Nov;104(11):783-791.
- 33. Donato F, Boffetta P, Puoti M. A meta-analysis of epidemiological studies on the combined effect of hepatitis B and C virus infections in causing hepatocellular carcinoma. Int J Cancer 1998 Jan;75(3):347-354.
- 34. Crespo J, Lozano JL, de la Cruz F, Rodrigo L, Rodríguez M, San Miguel G, et al. Prevalence and significance of hepatitis C viremia in chronic active hepatitis B. Am J Gastroenterol 1994 Aug;89(8):1147-1151.
- 35. Kao JH, Chen PJ, Lai MY, Chen DS. Occult hepatitis B virus infection and clinical outcomes of patients with chronic hepatitis C. J Clin Microbiol 2002 Nov;40(11):4068-4071.
- 36. Cacciola I, Pollicino T, Squadrito G, Cerenzia G, Orlando ME, Raimondo G. Occult hepatitis B virus infection in patients with chronic hepatitis C liver disease. N Engl J Med 1999 Jul;341(1):22-26.
- 37. Chen HY, Su TH, Tseng TC, Yang WT, Chen TC, Chen PJ, et al. Impact of occult hepatitis B on the clinical outcomes of patients with chronic hepatitis C virus infection: A 10-year follow-up. J Formos Med Assoc 2017 Sep;116(9):697-704.
- 38. Pontisso P, Ruvoletto MG, Fattovich G, Chemello L, Gallorini A, Ruol A, et al. Clinical and virological profiles in patients with multiple hepatitis virus infections. Gastroenterology 1993 Nov;105(5):1529-1533.
- 39. Sagnelli E, Coppola N, Scolastico C, Mogavero AR, Filippini P, Piccinino F. HCV genotype and “silent” HBV coinfection: two main risk factors for a more severe liver disease. J Med Virol 2001 Jul;64(3):350-355.