Introduced in 2013, sodium-glucose cotransporter type 2 inhibitors (SGLT2i) are a relatively new class of drugs known for their glycemic and weight reduction effects, cardiovascular disease (CVD) risk reduction in type 2 diabetes mellitus (T2DM) patients, and renal protective effects in patients with chronic kidney disease. However, there are emergent risks associated with SGLT2i use. These include euglycemic diabetic ketoacidosis (eDKA), bone fractures, acute renal injury, Fournier’s gangrene, and lower limbs amputations.1
In clinical trials with SGLT2i, eDKA rates ranged between 0.2 to 0.8 cases per 1000 patient-years among T2DM patients.2 The tendency for underlying eDKA to accompany normal or near-normal glucose levels (< 13.9 mmol/L) may delay its recognition and management.1 Precipitants of overt DKA include surgery, extensive exercise, myocardial infarction, stroke, severe infections, prolonged fasting, and other stressful physical and medical events.2 Here, we described the case of a 26-year-old male patient with poorly controlled T2DM on multiple oral hypoglycemic agents, who developed life-threatening severe eDKA associated with SGLT2i use (canagliflozin). The acidosis was prolonged and refractory to standard DKA therapy and renal replacement therapy, but was managed effectively with insulin titration based on capillary ketone measurements.
Case Report
A 26-year-old male presented to our hospital’s emergency department in January 2020 with a four-hour history of agitation, shortness of breath, and abdominal pain. This was preceded by four days of fatigue, malaise, fever, and sore throat. He had recently joined the national military service. He was diagnosed with T2DM for seven years and was being treated in another institute and maintained on multiple oral hypoglycemic agents of which he could recall only metformin by name. He reported no history of recent travel. Physical examination was remarkable for dry oral mucosa. He was in distress, febrile (38.5 oC), tachycardic (110 beats per minute), tachypneic (28 breaths per minute), and had low blood pressure (110/50 mmHg) despite having received 2 L of normal saline prior to presentation. The patient was also morbidly obese with BMI of 41.52 kg/m2 (120 kg; 170 cm). He had non-exudative pharyngitis. Chest examination revealed shallow deep breathing and bilateral vesicular breathing without added sounds. Cardiac exam was normal apart from sinus tachycardia. There was mild epigastric tenderness and other systemic examination was unremarkable.
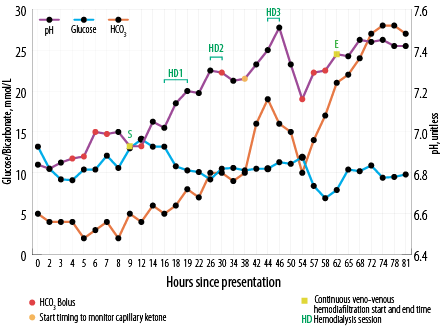
Figure 1: Changes in blood glucose, bicarbonate (HCO3), and pH since presentation and timings of renal replacement therapy.
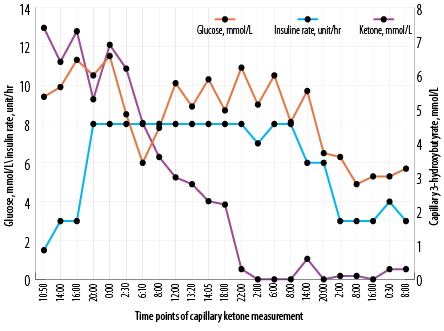
Figure 2: Insulin infusion rates compared with the levels of glucose and capillary ketones.
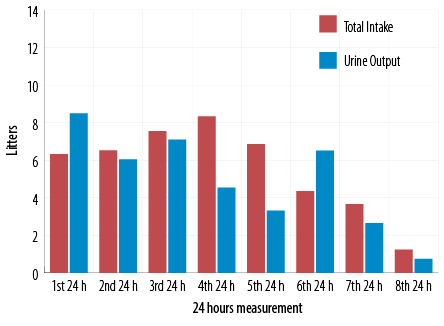
Figure 3: Total fluid intake and urine output.
Initial investigations revealed mild hyperglycemia (13.6 mmol/L), severe metabolic acidosis with bicarbonate level of 4 mmol/L, venous blood pH of 6.84, and high anion gap of 34. Urine ketone level was high (15 mmol/L) while lactic acid was normal at 2 mmol/L. The patient also had leukocytosis with white blood count of 29 × 109/L, hemoglobin of 16.4 g/L and platelet count of 350 × 109/L. His C-reactive protein at 64 (≤ 5) g/L, and procalcitonin at 2.3 (≤ 0.5) ng/mL were at elevated levels. The toxicology screen for alcohol and illicit drugs was negative. The following were within normal limits: sodium (135 mmol/L), potassium (3.8 mmol/L), creatinine (85 μmol /L), and urea (4 mmol/L). He had no rhabdomyolysis with normal total creatinine kinase of 223 IU/L. Serum osmolality was elevated at 315 mOsm/kg. Liver function and thyroid tests were normal.
Based on the above, broad-spectrum antibiotic therapy was started and diabetic ketoacidosis protocol was initiated with 10 units of insulin bolus followed by insulin infusion. Despite another five liters of crystalloid fluid, the patient’s pH remained the same. Therefore, sodium bicarbonate (NaHCO3) was infused intermittently (initial total of 250 mL of 8.4% NaHCO3; nonetheless pH remained static around 6.99 [Figure 1]. Within a few hours of presentation, the patient developed respiratory distress and required assisted ventilation. As recommended by the nephrology specialist, renal replacement therapy (RRT) was initiated.
Point of care inpatient ketone measurement facility was not available in our hospital and the insulin rate was titrated based on serial glucose measurements which was well controlled with insulin infusion at the rate of one unit per hour. Basal insulin was started on the second day of admission along with insulin infusion.
After 18 hours from presentation, the patient’s family brought in his home medications, which included daily gliclazide 120 mg, canagliflozin 300 mg, pioglitazone 30 mg, liraglutide 1.8 mg injection, and metformin/sitagliptin (1000/50 mg) twice daily. It was reported that he had uncontrolled diabetes and had refused to start insulin despite his primary physician’s recommendations. Later, it was revealed that he was on canagliflozin (an SGLT2i) 300 mg daily for the preceding three years.
Thirty-eight hours after presentation, serial capillary blood ketone measurements using the FreeStyleOptium ketone meter (Precision Xceed!; Abbott Diabetes Care, Maidenhead, UK) was initiated to titrate insulin infusion rate along with escalation of both dextrose and intravenous potassium replacement aiming for ketosis clearance [Figure 2]. The patient received a total of three sessions of intermittent hemodialysis (three hours each) and continuous veno-venous hemodiafiltration (CVVHDF) at 30 mL/kg/hour starting before first dialysis session and continued in between and after the last dialysis session [Figure 1]. In between, he required more NaHCO3 boluses as well [Figure 1]. It took 62 hours to correct metabolic acidosis and thereby close the anion gap by means of RRT and changing the base of titration of insulin rate to capillary ketone levels.
On day four of hospitalization, the patient was extubated. On day five, he was shifted to basal bolus regimen. Though eDKA had resolved, ketonemia (> 0.6 mmol/L) was persistent till day 12. Prior to knowing his home medications, and in view of the refractory nature of the severe metabolic acidosis, metabolic team was consulted to rule out the possibility of inborn metabolic errors. Urine organic acid profile revealed peaks of 2-ketoisovaleric acid, acetoacetic acid, 3-OH butyric acid, and 2-OH isovaleric acid, suggestive of ketosis and advanced catabolism. In addition to that, he was noticed to have significant diuresis during the first 24 hours of presentation [Figure 3].
Further investigations including blood, sputum, and urine cultures were negative. Influenza screen, Strep A, HIV, and hepatitis B and C were also negative. Respiratory viral panel MDX was positive for adenovirus. Chest x-ray was normal initially. HbA1C was 13.3%. C-peptide was 0.11 nmol/L, Anti IA2, GAD antibodies, and insulin antibodies were negative.
The patient’s hospital stay was complicated with provoked femoral line site deep venous thrombosis and pulmonary embolism which was managed with anticoagulants. At discharge, he had spent 16 days in hospital.
Discussion
SGLT2i are a class of oral glucose-lowering agents, first marketed in 2013. Thereafter, many reports emerged suggesting that SGLT2i increased the risk of eDKA. In 2015, US Food and Drug Administration (FDA) issued a drug safety communication that warned of an increased risk of eDKA associated with all SGLT2i class of drugs.3 The FDA also identified potential eDKA triggering factors such as intercurrent illness, lower food and fluid intake, reduced insulin doses, and history of alcohol consumption.
A study using a large claims database in the USA found that the incidence of DKA within 180 days following initiation of SGLT2i was 2.2-fold higher than with dipeptidyl peptidase-4 inhibitors, the latter of which have no known association with eDKA.4 From reports to the US FDA’s Adverse Event Reporting System, Fadini et al,5 found that higher proportional reporting ratios of 7.9 (95% CI: 7.5–8.4) for DKA in reports that mentioned SGLT2i compared to those without SGLT2i and having a diabetes indication and was higher for type 1 diabetes.
In cardiovascular outcome trials (CVOTs), the proportion of patients with reported diabetic ketoacidosis was similar in the SGLT2i and placebo groups for empagliflozin (EMPA-REG OUTCOME) and canagliflozin (CANVAS program), but it was higher for dapagliflozin (DECALRE-TIMI 58) with hazard ratio of 2.18 (95% CI: 1.10–4.30; p = 0.02).1
Limenta et al,6 provided demographic and baseline laboratory investigation profiles of 20 DKA cases associated with SGLT2i that had been reported to the Health Sciences Authority, Singapore. Apart from the difference in blood glucose levels, there were no noted differences in the profiles of typical and euglycemic DKA cases. However, no data was provided regarding the duration of DKA resolution and intensive care unit (ICU) stay.6
A recent Korean study reported that patients using SGLT2i needed longer ICU stays than non-users (4 days vs. 2 days; p = 0.019). It also showed that on average DKA episodes developed after 124 days (range: 7–380 days) of starting SGLT2i.7
Several retrospective studies reported that in the absence of SLGT2i, biochemical resolution of DKA took place in 11–12 hrs.8 The longer duration of SLGT2i-associated DKA is not emphasized in current management guidelines and has not been widely described in the literature. One reason could be that the majority of reported cases were managed successfully with standard DKA protocol and the time taken for DKA resolution was less of a research priority.
On the other hand, the impact of morbid obesity on DKA outcomes has been highlighted by a few retrospective studies. Elsheikh et al,9 reported increased mortality associated with morbid obesity in patients with DKA with an adjusted odds ratio (AOR) of 1.37 (95% CI: 1.18–1.61, but not obesity with lower BMI in the range 30–40.9 Similarly, Mudgal et al,10 large-scale study found that morbidly obese DKA patients had higher mortality than those without morbid obesity (0.72% vs. 0.38%; AOR = 1.85; p = 0.040). The study also reported that obese DKA patients had longer hospital stays (3.79 vs. 3.14 days, p < 0.001) than their non-obese counterparts.
It is worth mentioning that in the Korean study, there was no difference in BMI between SGLT2i users and nonusers.7
In Table 1, we have summarized seven reported cases with characteristics similar to ours. Like in those cases, our patient’s insulin infusion rate was high and the duration prolonged. Ketones are cleared faster if the insulin infusion rate is based on serum ketone levels rather than on serum glucose. In the present case this shift was made at a later stage. Early adoption of this, strategy will also prevent recurrence of ketoacidosis in the recovery phase.
Table 1: Reported cases of severe prolonged euglycemic diabetic ketoacidosis(eDKA) with sodium glucose cotransporter type 2 inhibitors (SGLT2i) use and modalities of treatment.
|
Current report |
26 y/o, male
T2DM for seven years + multiple OHAs (poorly controlled) |
Canagliflozin 300 mg |
Infection |
41.5 |
13.6 |
4.0 |
34.0 |
Urine ketone 3+ (15) |
6.84 |
Insulin infusion + NaHCO3 + 3 hemodialysis sessions + CVVHDF |
Intubation+
Ketonemia persisted for total 12 days |
62 hours |
|
Sloan et al,
201811 |
63 y/o, male
T2DM for 23 years
+ on mixed insulin |
Canagliflozin
seven months before presentation |
Silent myocardial infarction and diverticulitis |
27.2 |
13.3 |
8.0 |
– |
5.2 |
7.15 |
Fixed and variable insulin infusion rates |
Ketonemia persisted for total 12 days |
five days of IV insulin |
|
Maadarani et al, 201612 |
44 y/o, male
T2DM for eight years + insulin glargine and glimepiride |
Dapagliflozin 5 mg
one month before presentation |
No identifiable factor |
31.0 |
7.9 |
6.0 |
35.0 |
10.0 |
7.01 |
Insulin infusion up to 10 units/hour + NaHCO3 + eight hours CVVH |
. |
48 hours |
|
Gelaye et al, 201613 |
54 y/o, male
T1DM + insulin glargine |
Canagliflozin 300 mg
three years before presentation |
Postoperative day 2
laparoscopic appendectomy |
– |
7.9 |
9.0 |
37.0 |
12.4 |
7.06 |
Insulin infusion up to 10 units/hour + NaHCO3 infusion + hemodialysis |
Intubation + Fomepizole |
72 hours |
|
Rafey et al, 201914 |
44 y/o, male
T2DM for five years+ GLP-1 agonist
(poorly controlled) |
Canagliflozin 300 mg |
Postop day 6
post C5-C7 cervical decompression |
38.8 |
9.4 |
44.8 |
33.8 |
4.3 |
7.10 |
Insulin infusion + basal insulin |
|
92 hours |
|
Rafey et al, 201914 |
59 y/o, female
T2DM for 17 years + basal bolus insulin
(poorly controlled) |
Empagliflozin
25 mg
five months before presentation |
Postop day 3
elective laparoscopic right partial nephrectomy |
39.0 |
12.3 |
9.3 |
32.0 |
4.8 |
7.23 |
Insulin infusion |
Stopping DKA protocol after 28 hours after normalization of glucose and pH lead to relapse |
92 hours |
|
Nappi et al, 201915 |
67 y/o, female T2DM for five years +
metformin |
Empaglilozin 25 mg
one month before presentation with low calories intake |
NA |
21.5 |
16.6 |
1.8 |
31.0 |
After 12 hours urine ketone 80 mg/dL |
6.91 |
Insulin infusion +
NaHCO3 + two days
hemodialysis |
|
1 week |
T2DM: Type 2 diabetes mellitus; OHAs: Oral hypoglycemic agents; T1DM: Type 1 diabetes mellitus; GLP-1: Glucagon-like peptide 1; CVVH: Continuous veno-venous hemofiltration; CVVHDF: Continuous veno-venous hemodiafiltration;
CRRT: Continuous renal replacement therapy.
As in the other cases in Table 1, RRT was required for our patient. Whether to base the titration of insulin infusion rate on serum ketones from the start of the DKA protocol—as it may correct the acidosis faster and hold the need for RRT—is worth considering for future cases. However, in previous reported cases, despite delivering insulin at rates as high as 10 units/h, RRT was still needed. Such persistently high acidosis may be due to the action of SGLT2i, which is implicated in multiple pathophysiological processes, such as reduced pancreatic insulin secretion, increased glucagon secretion by direct stimulation of alpha pancreatic cells, increased conversion of fatty acids to ketone bodies by beta-oxidation in the liver (lipolysis), as well as increased ketone and acetoacetate reabsorption in renal tubules leading to reduced renal ketone excretion.17
Conclusion
With the expanding list of indications and use of SLGT2i, it is worth being aware of the special characteristics of DKA in presence of this class of drugs, and the importance of rapid clearance of ketosis by means of higher insulin infusion rates linked to the patient’s serum ketone levels.
Disclosure
The authors declare no conflicts of interest. Written consent was obtained from the patient.
references
- 1. McGill JB, Subramanian S. Safety of Sodium-Glucose Co-Transporter 2 Inhibitors. Am J Cardiol 2019 Dec;124(Suppl 1):S45-S52.
- 2. Handelsman Y, Henry RR, Bloomgarden ZT, Dagogo-Jack S, DeFronzo RA, Einhorn D, et al. American association of clinical endocrinologists and American college of endocrinology position statement on the association of SGLT-2 inhibitors and diabetic ketoacidosis. Endocr Pract 2016 Jun;22(6):753-762.
- 3. Rosenstock J, Ferrannini E. Euglycemic diabetic ketoacidosis: A predictable, detectable, and preventable safety concern with sglt2 inhibitors. Diabetes Care 2015 Sep;38(9):1638-1642.
- 4. Fralick M, Schneeweiss S, Patorno E. Risk of Diabetic Ketoacidosis after Initiation of an SGLT2 Inhibitor. N Engl J Med 2017 Jun 8;376(23):2300-2302.
- 5. Fadini GP, Bonora BM, Avogaro A. SGLT2 inhibitors and diabetic ketoacidosis: data from the FDA Adverse Event Reporting System. Diabetologia 2017 Aug;60(8):1385-1389.
- 6. Limenta M, Ho CS, Poh JW, Goh SY, Toh DS. Adverse Drug Reaction Profile of SGLT2 Inhibitor-Associated Diabetic Ketosis/Ketoacidosis in Singapore and their Precipitating Factors. Clin Drug Investig 2019 Jul;39(7):683-690.
- 7. Jeon JY, Kim SK, Kim KS, Song SO, Yun JS, Kim BY, et al; DKA Study Group of Gyeonin Branch of the Korean Diabetes Association. Clinical characteristics of diabetic ketoacidosis in users and non-users of SGLT2 inhibitors. Diabetes Metab 2019 Oct;45(5):453-457.
- 8. Lee MH, Calder GL, Santamaria JD, MacIsaac RJ. Diabetic ketoacidosis in adult patients: an audit of factors influencing time to normalisation of metabolic parameters. Intern Med J 2018 May;48(5):529-534.
- 9. Elsheikh A, Abdulla A, Wahab A, Eigbire G, Salam A, Rajamani K. Impact of Obesity on Outcomes of Diabetic Ketoacidosis—Results from the National Inpatient Sample. Diabetes 2018 Jul; 67(Supp1):2085.
- 10. Mudgal M, Goel A. 537-P: Impact of Obesity on Outcomes of Diabetic Ketoacidosis: A Nationwide Analysis. Diabetes 2019 Jun;68(Supplement 1).
- 11. Sloan G, Kakoudaki T, Ranjan N. Prolonged diabetic ketoacidosis associated with canagliflozin. Endocrinol Diabetes Metab Case Rep 2018 Jun;2018:1-5.
- 12. Maadarani O, Bitar Z, Alhamdan R. Dapagliflozin- Induced Severe Ketoacidosis Requiring Hemodialysis. Clin Med Rev Case Rep 2016;3:150.
- 13. Gelaye A, Haidar A, Kassab C, Kazmi S, Sinha P. Severe Ketoacidosis Associated with Canagliflozin (Invokana): A Safety Concern. Case Rep Crit Care 2016;2016:1656182.
- 14. Rafey MF, Butt A, Coffey B, Reddington L, Devitt A, Lappin D, et al. Prolonged acidosis is a feature of SGLT2i-induced euglycaemic diabetic ketoacidosis. Endocrinol Diabetes Metab Case Rep 2019 Sep;2019(1):6-10.
- 15. Nappi F, La Verde A, Carfora G, Garofalo C, Provenzano M, Sasso FC, et al. Nephrology consultation for severe SGLT2 inhibitor-induced ketoacidosis in type 2 diabetes. Medicina (Kaunas) 2019 Aug;55(8):4-9.
- 16. Yeo SM, Park H, Paek JH, Park WY, Han S, Park SB, et al. Ketoacidosis with euglycemia in a patient with type 2 diabetes mellitus taking dapagliflozin: A case report. Medicine (Baltimore) 2019 Jan;98(3):e14150.
- 17. Qiu H, Novikov A, Vallon V. Ketosis and diabetic ketoacidosis in response to SGLT2 inhibitors: Basic mechanisms and therapeutic perspectives. Diabetes Metab Res Rev 2017 Jul;33(5):1-10.