Quality of life (QoL) refers to anindividual’s self-perception over a range of life domains (e.g., physical abilities, mental health, social relationships, sense of life fulfillment).1–4 In other words, QoL covers how an individual measures the ‘goodness’ of numerous aspects of their life.4
As QoL is a subjective multi-dimensional construct, its measurement requires using various indicators (e.g., health status, personal functioning).1,5 Instruments to measure QoL can be divided into three categories.2 First, there are generic instruments for the general population, such as the 36-item short-form health survey (SF-36).2,4,6 Such tools assess a wide variety of generic domains (e.g., physical function, energy, and vitality).2 Second, there are disease-specific instruments, such as kidney disease quality of life (KDQOL).2,4,7 Lastly, there are symptom-specific measures that emphasize symptoms associated with a particular disease and its treatment (e.g., duration of recovery after a dialysis session).2 Thus, QoL can be used to gauge health system performance, mortality indicators, and comparison of health between groups.
QoL has been found to be a consistent and strong predictor of health outcomes among patients suffering from end-stage renal disease (ESRD).8 The present review focused on adult ESRD patients whose treatment process is challenging and prolonged. Our special focus was on the management of patient QoL, because ESRD, the fifth and final stage of chronic kidney disease (CKD), requires lifelong renal replacement therapy (RRT) (e.g., dialysis, and kidney transplantation).9–12 The two leading causes of ESRD worldwide are diabetes which affects 5–8% of adults, and hypertension, prevalent among 1.3 billion adults.13,14 Despite the extensive resources committed to treating ESRD and the significant improvements in the quality of hemodialysis (HD) over the years, patients continue to experience morbidity and poor QoL.13,15 ESRD patients are required to restrict their fluid intake and at the same time, they are at risk of suboptimal nutrition.8 They are also at high risk for cardiovascular disease and mortality.8,12,16 Consequently, patients with ESRD tend to suffer from severe psychological distress.10,15 Depression accompanied by anxiety is very common among ESRD patients.15,16 Between patients undergoing chronic HD while feeling depressed and those undergoing HD without depression, the former have been found twice as likely to die or require hospitalization within a year. Depressed ESRD patients may also carry a 30% higher risk for multiple hospitalizations and longer accumulative admission days. Consequently, ESRD patients experience significant functional impairment and decreased QoL.10,15
Ever since the implementation of HD in the 1960s, nephrologists have been aware of the impaired QoL of patients with ESRD.2 Currently, optimizing patient care to maximize patients’ QoL is a key outcome goal.5 Here, psychological interventions are increasingly being considered to hold promise.5,8,15,16 Psychological intervention can be defined as referral to a mental health clinic, providing psychotherapy of various types, and prescribing psychiatric medications if necessary.15 For ESRD patients, however, the latter option calls for abundant caution due to limited risk data.14,15 Certain categories of psychiatric drugs are known to carry higher risks for ESRD patients (e.g., high doses of benzodiazepines could cause sedation).17
Thus, safer QoL interventions such as psychotherapy are being explored more.15 These include cognitive behavioral therapy (CBT) and exercise therapy.16,18 Considerable number of studies evaluate CBT positively.16,18,19 Patients who underwent CBT have reported reduced emotional distress and increased adherence to fluid restrictions. Regarding exercise therapy, mixed results were reported.16 While some studies endorsed the efficacy of exercise therapy,19,20 others revealed no significant changes.21,22
Despite the increasing focus on QoL of ESRD patients,23 research is still scarce on the effectiveness of psychotherapy as a QoL booster.8,24 To our knowledge, there are no systematic reviews or meta-analyses on this topic. That research gap is being narrowed by our current study—a systematic review cum meta-analysis which evaluates previous research from different parts of the world that studied the effects of various psychotherapeutic interventions on QoL among patients with ESRD.
Methods
Protocol registration
This review was registered in the Malaysian National Medical Research Register (No. NMRR-20-881-54565) and approved by Sunway Medical Centre Independent Research Ethics Committee (No: SREC 005/2017/ER). The review was conducted on the basis of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.25
Literature search
Two investigators (K.P. and A.D.N.) independently conducted electronic searches for potential studies via MEDLINE, PubMed, and SAGE Journals on 10 April 2020. The search yielded relevant studies published in various journals from inception to 10 April 2020.
Search strategies
The search term combinations used were: (psychotherapy* OR cognitive therapy* OR cognitive psychotherapy* OR brief psychotherapy* or short*term psychotherapy* OR group psychotherapy* OR psychodynamic psychotherapy* OR rational psychotherapy* OR rational*emotive psychotherapy*) (quality of life OR health*related quality of life) AND (kidney disease OR chronic kidney disease OR renal insufficiency OR chronic renal insufficiency OR kidney failure OR chronic kidney failure OR renal disease OR chronic renal disease OR end*stage renal disease). The search strategies are presented in Appendix 1.
Intervention and control group definitions
The term intervention group refers to participants in a study who received any form of psychotherapy during the intervention period. The control group refers to participants who did not receive any form of psychotherapy during the intervention period or thereafter.
Study screening
Relevant studies identified through the database searches were imported into Mendeley 1.19.4, a document management software. Duplicates were removed. The titles and abstracts of the remaining articles were screened based on the search strategies. The full-text articles found were assessed based on the inclusion criteria mentioned below. In addition, reverse-forward citation tracking was conducted manually from the identified studies. All steps were independently carried out by two investigators (K.P. and A.D.N.). If any discrepancy arose between studies selected by the two investigators, discussion was held and resolved by the senior authors (K.W.L. and P.B.O.) for final consensus before the full text of each relevant article was reviewed.
Study selection
The selection criteria required studies with randomized controlled trial design on patients with ESRD. Only studies published in English language, with full-text content available, were considered suitable. Studies that did not meet these criteria were excluded.
Data extraction
The following characteristics were extracted from the selected studies: last name of first author, year of publication, country, sample size, mean age± SD, gender, kidney disease stage, measure/s, type of intervention, duration of intervention, information from intervention and control groups (baseline mean ±SD, final mean±SD, and mean difference±SD), p-value for difference in mean change between the two groups, and the risk of bias. Two investigators (K.P. and A.A.A.L.) extracted the data individually, and differences were resolved through discussion with the third and fourth investigators (K.W.L. and P.B.O.).
Statistical analysis
Mean differences in QOL between groups were calculated using random-effects meta-analysis. This was carried out using Review Manager 5 (RevMan 5.3),26 whereby weighted mean difference (WMD)was subjected to a two-tailed test to yield a statistically significant p-value of < 0.050. To assess heterogeneity between the studies, I2 index was examined. Besides that, publication bias was assessed using funnel plots, Egger’s test, and Begg’s test via Meta-Essentials.27
Risk of bias assessment
Two reviewers (K.P. and A.A.A.L.) used the Revised Cochrane risk-of-bias tool for randomized trials to independently assess the risk of bias within each study.28 All sources of bias (e.g., randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, and selection of the reported result) were evaluated accordingly. Conflicts were resolved by discussing with the third and fourth investigators (K.W.L. and P.B.O.).
Ethical approval was obtained from the Malaysian National Medical Research Register (NMRR) (NMRR-20-881-54565) and Sunway Medical Centre Independent Research Ethics Committee (SREC) (SREC 005/2017/ER). Funding was granted by Sunway University Internal Grant Scheme (GRTIN-RSF-SHMS-DMS-02-2020).
Results
Description of included studies
The literature search and selection process are presented in Figure 1.
The literature search initially identified 149 articles. After excluding 39 duplicates, 110 studies were retrieved to review the titles and abstracts. From these, 25 studies were considered eligible to undergo full-text assessment for inclusion criteria. After a comprehensive evaluation of these articles, eight studies were finally selected for systematic review and meta-analysis.
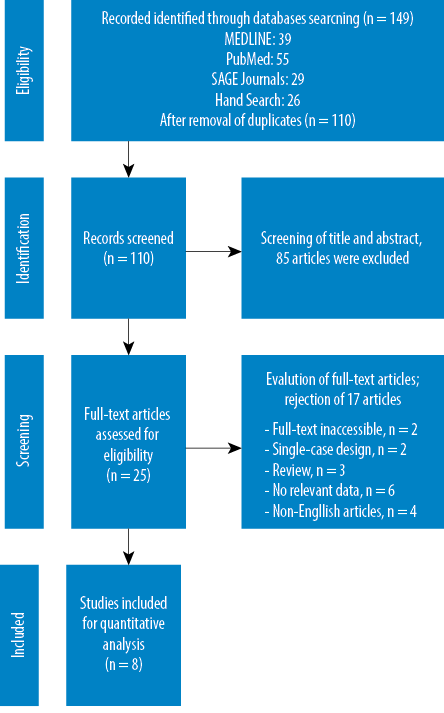
Figure 1: Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram of the literature screening process.
Characteristics of the studies
The characteristics of the eight studies are summarized in Table 1.
The subjects of the current systematic review and meta-analysis comprised of 448 kidney disease patients (239 men and 209 women) from across the eight selected studies. Three studies had been conducted in the USA,29,31,35 two in Taiwan,33,36 two in Iran,32,34 and one in Brazil.30 Only five papers reported the mean ages of their participants.30,31,34–36
The studies used different measures to assess QoL, SF-36 being the most common,33,35,36 followed by KDQOL-SF,29,30 Ferrans and Powers quality of life questionnaire,32,34 KDQOL-36,31 and quality of life inventory (QOLI).35 Regarding therapeutic interventions, three studies applied CBT,29,30,33 while the others used problem-solving therapy (PST),31 self-care education,32 empowerment intervention,34 quality of life therapy (QOLT),35 and adaptation training program (ATP).36 In addition, in half of the studies, the average duration of intervention was two months.2,33,35
For meta-analysis, we included only five studies.30,31,33,35,36 Specifically, the mean differences in physical component summary (PCS) and mental component summary (MCS) scores from the KDQOL-SF, KDQOL-36, and SF-36 were examined to attain a direct picture of QoL. These subscale outcomes were selected because previous studies found a significant relationship between these subscale scores and their respective patient outcomes.7,31,37,38 Furthermore, factor analysis studies have demonstrated two distinct groupings for QoL, namely the PCS and MCS.33,39
Table 1: Characteristics of the included eight studies.
|
Cukor et al,29 |
2014 |
USA |
65 |
N/A |
N/A |
47 |
18 |
KDQOL-SF |
CBT |
3 months |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
Low risk |
|
Duarte et al,30 |
2009 |
Brazil |
85 |
52.4 ± 15.9 |
54.0 ± 12.7 |
35 |
50 |
KDQOL-SF |
CBT |
3 months |
1 ± 9.6 |
0.58 |
–0.80 ± 8.8 |
0.60 |
9.9 ± 12.3 |
< 0.001 |
-1.8 ± 11.7 |
0.45 |
0.24 |
0.002 |
Some concerns |
|
Erdley-Kass et al,31 |
2018 |
USA |
33 |
72.27 ± 5.62 |
75.33 ± 8.29 |
21 |
12 |
KDQOL-36 |
PST |
1.5 months |
3.02 ± 11.07 |
N/A |
–1.57 ± 8.53 |
N/A |
10.41 ± 10.23 |
N/A |
-0.81 ± 9.72 |
N/A |
0.27 |
0.020 |
Low risk |
|
Ghadam et al,32 |
2016 |
Iran |
50 |
N/A |
N/A |
26 |
24 |
Ferrans and Powers QoL Questionnaire |
Self-care education |
2 months |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
High risk |
|
Lii et al,33 |
2007 |
Taiwan |
48 |
N/A |
N/A |
23 |
25 |
SF-36 |
CBT, self-efficacy theory |
2 months |
2.55 ± 6.67 |
N/A |
–2.96 ± 6.76 |
N/A |
3.52 ± 7.38 |
N/A |
0.25 ± 9.05 |
N/A |
0.008 |
0.19 |
Low risk |
|
Moattari et al,34 |
2012 |
Iran |
48 |
38.56 ± 11.4 |
37.3 ± 12.79 |
31 |
17 |
Ferrans and Powers QoL Questionnaire |
Empower-ment interve-ntion |
1.5 months |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
Some concerns |
|
Rodrigue et al,35 |
2011 |
USA |
62 |
53.2 ± 11.1 |
52.7 ± 12.7 |
29 |
33 |
QOLI, SF-36 |
QOLT |
2 months |
–0.1 ± 6.2 |
N/A |
–1.7 ± 10.4 |
N/A |
5.5 ± 9.6 |
N/A |
0.4 ± 15.3 |
N/A |
0.36 |
0.13 |
Low risk |
QoL: quality of life; PCS: physical component summary; MCS: mental component summary; N/A: not available; KDQOL-SF: Kidney Disease Quality of Life Short Form; KDQOL-36: Kidney Disease Quality of Life; SF-36: 36-Item Short Form Health Survey; QOLI: Quality of Life Inventory; CBT: cognitive-behavioral therapy; PST: problem-solving therapy; QOLT: quality of life therapy; ATP: adaptation training program.
Effects of psychotherapy on quality of life and its subgroup analysis
The effect of psychotherapy on QoL by PCS and MCS subgroup analysis are presented in Tables 2 and 3.
A statistically significant improvement in QoL (PCS) was observed in the intervention group (those who received psychotherapy) as compared to those in the control group (who received placebos) (WMD = 2.52, 95% CI: 0.48–4.57) among all participants. Similar results were also seen in QoL (MCS) with (WMD) 4.22, 95% CI: 1.54–6.89. For both PCS and MCS, the heterogeneity of the studies was found to be I2 = 0%.
Table 2: Forest plot of the effects of psychotherapy on QoL (PCS) among ESRD patients.
|
Duarte et al,30 |
36.2 |
9.3 |
41 |
33.9 |
8.0 |
44 |
30.5 |
2.3 (-1.40–6.00) |
|
Erdley-Kass et al,31 |
37.1 |
11.1 |
15 |
33.8 |
8.5 |
18 |
8.9 |
3.28 (-3.57–10.13) |
|
Lii et al,33 |
42.9 |
5.9 |
20 |
40.5 |
9.8 |
28 |
21.1 |
2.41 (-2.04–6.86) |
|
Rodrigue et al,35 |
3.4 |
6.6 |
22 |
35.2 |
9.5 |
20 |
16.7 |
1.20 (-3.79–6.19) |
|
Tsay et al,36 |
43.9 |
5.8 |
30 |
40.3 |
9.9 |
27 |
22.9 |
3.60 (-0.67–7.87) |
QoL: quality of life; PCS: physical component summary; ESRD: end-stage renal disease ; IV: Intervention.
Heterogeneity: Tau2 = 0.00; Chi2 = 0.58, df = 4 (p = 0.97); I2 = 0%; test for overall effect: Z = 2.42 (p = 0.02).
Table 3: Forest plot of the effects of psychotherapy on QoL (MCS) among ESRD patients.
|
Duarte et al,30 |
47.3 |
12.1 |
41 |
39.3 |
11.9 |
26 |
20.7 |
8.00 (2.11, 13.89) |
|
Erdley-Kass et al,31 |
54.1 |
10.2 |
15 |
51.4 |
9.7 |
18 |
15.3 |
2.71 (-4.14, 9.56) |
|
Lii et al,33 |
43.5 |
7.5 |
20 |
40.1 |
12.1 |
28 |
23.1 |
3.39 (-2.17, 8.95) |
|
Rodrigue et al,35 |
46.2 |
11.3 |
22 |
42.8 |
12.0 |
20 |
14.3 |
3.40 (-3.67, 10.47) |
|
Tsay et al,36 |
44.0 |
7.2 |
30 |
40.7 |
11.9 |
27 |
26.6 |
3.30 (-1.89, 8.49) |
QoL: quality of life; PCS: physical component summary; ESRD: end-stage renal disease; IV: intervention.
Heterogeneity: Tau2 = 0.00; Chi2 = 2.03; df = 4 (p = 0.73); I2 = 0%; Test for overall effect: Z = 3.09 (p = 0.002).
Risk of bias within studies
The risk of bias was examined under five domains, including randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, and selection of the reported result. The results are shown in Appendices 2 and 3.
Randomized controlled trials suggested that all the eight studies had low risk of bias; the baseline differences between groups did not appear problematic in this respect. One study had a high risk of bias due to deviation from the intended intervention.32 There was a lack of data about addressing non-adherence circumstances (e.g., imperfect compliance by participants under treatment), which could have affected participants’ outcomes. The same study was also the only one with high risk of bias in the measurement of the outcome, as both the researcher and co-researcher participated in preparing the demographic questionnaire and in assessing the participant checklist, and it should be noted that the researchers were not blinded to the treatments.32 Another risk of the same study was lack of information on whether the data was analyzed in accordance with the pre-specified analysis plan or not, considering that only the p-value was reported.32
There was a low risk of missing outcome data as these were available for all participants. For selection of the reported result, some concerns were raised in two studies which provided no information on whether the numerical results were selected from multiple eligible analyses of the data or not.30,34 Further, in five studies no information was found on whether the numerical results were selected from multiple eligible analyses of the data or not. With that said, overall, the five studies were found have low risk of bias,29,31,33,35,36 two had some concerns,30,34 and one had high risk of bias.32
Publication bias analysis
The funnel plots of studies are shown in Figures 2 and 3.
For PCS, from the funnel plot, Egger’s test (p = 0.950) and Begg’s test (p = 1.000) suggested no publication bias in the studies. However, for the MCS component, as seen from the asymmetrical funnel plot, Egger’s test (p = 0.041) and Begg’s test (p = 0.014) yielded significant results, suggesting publication bias. Such results would also mean that significant asymmetry was found, and the results derived should be considered cautiously.
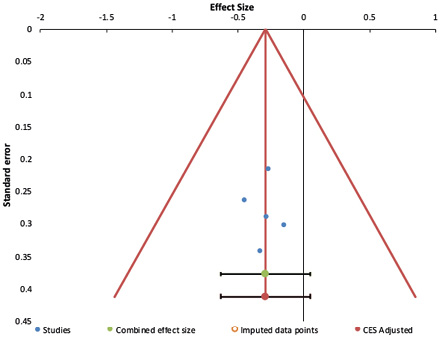
Figure 2: Funnel plot of studies evaluating the effects of psychotherapy on quality of life (physical component summary) among end-stage renal disease patients.
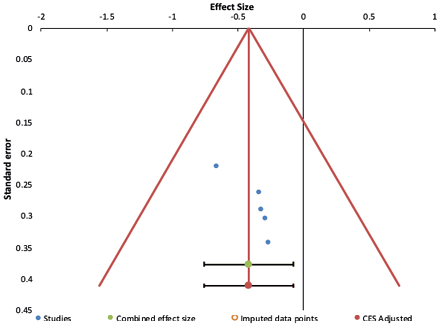
Figure 3: Funnel plot of studies evaluating the effects of psychotherapy on quality of life (mental component summary) among end-stage renal disease patients.
Discussion
The current study aimed to assess the effects of psychotherapy on QoL among ESRD patients through meta-analysis and systematic review.
From the meta-analysis of the selected five studies,30,31,33,35,36 we found that QoL (PCS and MCS) differed significantly between the intervention groups (those who received psychotherapy) and the control groups (those who did not). In other words, intervention groups displayed an overall significant increase in QoL compared to the controls. This suggests that psychotherapy may help improve the QoL of patients with ESRD. This includes improved physical and mental health aspects, as derived from the PCS and MCS respectively.31 It is known that ESRD is often negatively impacted, both physically (e.g., increased rates of hospitalizations) and psychologically (e.g., more depressed).10,15,31 Psychotherapeutic interventions have the potential to address both by combining cognitive restructuring and behavioral assignments.15,16,40,41 Thus, reduction of distorted thinking may enable acting pragmatically towards positive change, which in turn may improve QoL (physically and mentally) of the patients.15,16,18,19 Such improvements have also been observed the present meta-analysis.30,31,33,35,36
Our meta-analysis revealed that the physical (PCS) component of QoL in ESRD patients benefited the most from ATP.36 As ATP incorporates both transactional theory of stress and coping42 and CBT,40 it incorporates patient education, cognitive behavior modification, problem-solving, and stress management.36 The goal of ATP is to increase the patient’s sense of competence and mastery to initiate and reinforce constructive coping strategies. A reason for ATP’s efficacy in improving PCS-QoL could be that mainly physical difficulties were reported and managed in that particular study. The patient difficulties addressed included restrictions on fluid intake, length of dialysis treatment, loss of bodily function, ambulatory difficulties, and limitations in physical activities. As mentioned earlier, physical symptoms are among the main hurdles encountered by patients with ESRD.8 These prey on the mind of the patients who develop distorted views, further restricting their ability to cope.36 ATP method taught them to appraise these stressors realistically, then imparted factual knowledge about the disease process, and gave coping strategies to manage the physical difficulties. In short, ATP method appears to have provided both cognitive restructuring and physical coping skills to the patients, allowing them to adapt better to ESRD; thus, improving both the mental and physical components of their QoL.
Meanwhile, pure CBT was reported to significantly enhance the MCS compared to other types of psychotherapy.29,30,33 ESRD patients tend to develop distorted thinking (e.g., magnification), which trigger negative emotions such as feeling depressed, which may lead them to maladaptive behavior (e.g., reduced fluid adherence).16,30,41 CBT, with its focus on cognition, guides the client to rationally understand the undistorted reality of their difficulties and makes them aware of their distorted thoughts (e.g., how these thoughts trigger unpleasant emotions, how they engage in maladaptive behaviors to end the emotional discomfort). Once this cycle of thoughts and behavior is clear to them, the therapist goes on to suggest remedial thoughts that focus on positive action plans, which in turn enables the extinction of their previous maladaptive ‘thought-emotion-behavior’ pattern.16,30,40,41 Thus, in the three studies that used CBT, the patients were encouraged to talk about their thoughts, identify and restructure distorted thoughts, and apply coping strategies.30,40 In all three, improvements in the MCS component of QoL were evident.29,30,33 The patients reported reduction in pessimistic and negative thinking, whereby they were trained to apply positive alternate thoughts. With that, their emotions were
more relaxed.
Overall, various forms of psychotherapy helped improve the QoL of ESRD patients. However, the specific component of QoL that registered more benefit (PCS or MCS) depended on the type of psychotherapy used. ATP, for example, seems to benefit more in improving PCS,36 while CBT seems to enhance MCS.30,33
Implications of this study
Our study has identified the benefits of psychotherapy to improve the QoL among patients with ESRD. It indicates that healthcare providers should plan and implement management programs that include a suitable method of psychotherapy.5,8,14,16 Healthcare providers should also seek to increase the frequency and regularity of patient attendance for psychotherapeutic sessions, such as enhancing therapeutic alliance, offering convenient appointments, and providing reminders.43 This could allow room for patients to benefit from psychotherapy and thus, improving their QoL.
One category of patients among whom psychotherapy reaches less are recipients of donated kidneys. RRT is known to provide radical relief for end-stage kidney disease patients, limited only by the availability of kidney donors.9–12 The need for psychotherapeutic interventions for both RRT recipients and donors needs to be investigated and remedial measures taken.
Clinical significance of this Review
We selected five studies for meta-analysis. Two of these used CBT,30,33 while other studies used PST, QOLT, and ATP, respectively.31,35,36 For the PCS, ATP was found to produce the highest mean difference (MD) of 3.60,36 followed by PST (MD: 3.28),31 CBT (MD: 2.41; 2.30),30,33 and QOLT (MD: 1.20).35 For MCS, the highest MD was found where CBT was applied (MD: 8.00),33 followed by QOLT (MD: 3.40),35 ATP (MD: 3.30),36 and PST (MD: 2.71).31 In another study, when CBT was used, MD generated was 3.39.32
Based on the results obtained, various psychotherapeutic interventions improve both PCS and MCS in QoL to varying degrees. It must also be remembered that most benefited the mind (MCS) more than the body (PCS), with a possible exception of ATP.
As previously mentioned, ESRD patients encounter a number of physical hurdles such as fluid intake restrictions, dialysis treatment duration, and physical activity limitations.8 Here, ATP, with its dual focus on the body and the mind, helps initiate and reinforce new coping strategies, in turn enhancing the client’s sense of competence and self-mastery. Better cognitive restructuring and coping skills could allow better adaption and thus, improving the patients’ QoL, including the PCS.33 Thus, ATP appears to be a suitable intervention to promote the PCS aspect of QoL in ESRD patients.
As for MCS, it was observed that CBT seemed to fare better. ESRD patients tend to engage in distorted perceptions, thoughts, triggering strong emotions leading to impulsive and unhealthy coping behaviors that further feed the problem.16,33,41 CBT seeks to ‘decontaminate’ the cognitive mind of the patients so they perceive their problems with less distortion, and then help them to use the newly sharpened cognitive faculties to arrive at personalized coping strategies, leading to weakening of the habituated negative thoughts and emotions.16,33,40,41 Indeed, the benefits of CBT for QoL (MCS) have been supported by many studies.29,30,33 With such supportive evidence, CBT can be considered a preferred tool to mitigate the mental health concerns of patients with ESRD.
Strengths and limitations
To the authors’ knowledge, this is the first meta-analysis to examine the effects of psychotherapy on QoL among ESRD patients. In addition, the significant differences in QoL benefits between the intervention and control groups in all the eight studies strengthens the results of this study.
Several limitations were identified. The tools used in measuring QoL varied across studies. Further, different approaches were applied in measuring QoL, such as total scores,29,32,34,35 a number of dimensions (e.g., social-economical, family),32,33 and also as PCS and MCS.30,3133,35,36 Only five out of eight studies could be included in the meta-analysis because we had selected PCS and MCS as the key components of QoL. One reason for this choice was that our factor analysis results established the PCS-MCS duo as a distinct grouping that made up QoL.36,39 The importance of these two QoL components is supported by several studies which discovered a significant relationship between these subscale scores and their respective outcomes.7,31,37,38
Besides that, data of the present study were limited to a few countries, including USA, Taiwan, Iran, and Brazil. In addition, the studies from Iran had to be excluded from meta-analysis.32,34 With such limited data, a cross-cultural generalization would be difficult, requiring the results to be interpreted with caution. In addition, this review only included studies in English or at least with English abstracts. We also did not include non-published material which may not have been subjected to peer review. We may have difficulties to comprehend and assess non-English studies and any further translation exercise may affect its validity. Notwithstanding with these limitations, we found statistically significant improvement in QoL in those receiving psychotherapy compared to those in control group.
Conclusion
The current systematic review and meta-analysis found that psychotherapy helps to improve QoL in ESRD patients. Healthcare providers should continue to promote the inclusion of psychotherapeutic methods into their overall treatment plan for this population. Future studies should also explore this area further with a more diverse population, to allow broader and cross-cultural understanding and sufficient information for future clinical practice.
Disclosure
The authors declare no conflicts of interest.
Acknowledgements
The authors acknowledge the contributions of Akshina Dewi Nawoor in evolving search strategies and in the study selection process.
references
- 1. de la Torre-Luque A, Gambara H, López E, Cruzado JA. Psychological treatments to improve quality of life in cancer contexts: A meta-analysis. Int J Clin Health Psychol 2016 May-Aug;16(2):211-219.
- 2. Finkelstein FO, Arsenault KL, Taveras A, Awuah K, Finkelstein SH. Assessing and improving the health-related quality of life of patients with ESRD. Nat Rev Nephrol 2012 Dec;8(12):718-724.
- 3. Kolovos S, Kleiboer A, Cuijpers P. Effect of psychotherapy for depression on quality of life: meta-analysis. Br J Psychiatry 2016 Dec;209(6):460-468.
- 4. Theofilou P. RETRACTED: Quality of Life: Definition and Measurement. Europe’s. J Psychol 2013;9(1):150-162.
- 5. Moss AH, Davison SN. How the ESRD quality incentive program could potentially improve quality of life for patients on dialysis. Clin J Am Soc Nephrol 2015 May;10(5):888-893.
- 6. McDowell I. Measuring health: a guide to rating scales and questionnaires: Oxford University Press, USA; 2006.
- 7. Hays RD, Kallich JD, Mapes DL, Coons SJ, Carter WB. Development of the kidney disease quality of life (KDQOL) instrument. Qual Life Res 1994 Oct;3(5):329-338.
- 8. Kim J-Y, Kim B, Park K-S, Choi J-Y, Seo J-J, Park S-H, et al. Health-related quality of life with KDQOL-36 and its association with self-efficacy and treatment satisfaction in Korean dialysis patients. Qual Life Res 2013 May;22(4):753-758.
- 9. Caskey FJ, Kramer A, Elliott RF, Stel VS, Covic A, Cusumano A, et al. Global variation in renal replacement therapy for end-stage renal disease. Nephrol Dial Transplant 2011 Aug;26(8):2604-2610.
- 10. Palmer S, Vecchio M, Craig JC, Tonelli M, Johnson DW, Nicolucci A, et al. Prevalence of depression in chronic kidney disease: systematic review and meta-analysis of observational studies. Kidney Int 2013 Jul;84(1):179-191.
- 11. Ren Q, Shi Q, Ma T, Wang J, Li Q, Li X. Quality of life, symptoms, and sleep quality of elderly with end-stage renal disease receiving conservative management: a systematic review. Health Qual Life Outcomes 2019 May;17(1):78.
- 12. Stømer UE, Gøransson LG, Wahl AK, Urstad KH. A cross-sectional study of health literacy in patients with chronic kidney disease: Associations with demographic and clinical variables. Nurs Open 2019 Jul;6(4):1481-1490.
- 13. Couser WG, Remuzzi G, Mendis S, Tonelli M. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int 2011 Dec;80(12):1258-1270.
- 14. Zhou B, Carrillo-Larco RM, Danaei G, Riley LM, Paciorek CJ, Stevens GA, et al; NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. Lancet 2021 Sep;398(10304):957-980.
- 15. Hedayati SS, Yalamanchili V, Finkelstein FO. A practical approach to the treatment of depression in patients with chronic kidney disease and end-stage renal disease. Kidney Int 2012 Feb;81(3):247-255.
- 16. Shirazian S, Grant CD, Aina O, Mattana J, Khorassani F, Ricardo AC. Depression in chronic kidney disease and end-stage renal disease: similarities and differences in diagnosis, epidemiology, and management. Kidney Int Rep 2016 Sep;2(1):94-107.
- 17. De Sousa A. Psychiatric issues in renal failure and dialysis. Indian J Nephrol 2008 Apr;18(2):47-50.
- 18. Archibald D, Liddy C, Keely EJ. The Doctor Is (Virtually) In: Using Electronic Consultation to Provide Prompt Psychiatric Services. Psychiatr Serv 2018 Mar;69(3):362.
- 19. Chen H-Y, Chiang C-K, Wang H-H, Hung K-Y, Lee Y-J, Peng Y-S, et al. Cognitive-behavioral therapy for sleep disturbance in patients undergoing peritoneal dialysis: a pilot randomized controlled trial. Am J Kidney Dis 2008 Aug;52(2):314-323.
- 20. Barcellos FC, Santos IS, Umpierre D, Bohlke M, Hallal PC. Effects of exercise in the whole spectrum of chronic kidney disease: a systematic review. Clin Kidney J 2015 Dec;8(6):753-765.
- 21. Slinin Y, Greer N, Ishani A, MacDonald R, Olson C, Rutks I, et al. Timing of dialysis initiation, duration and frequency of hemodialysis sessions, and membrane flux: a systematic review for a KDOQI clinical practice guideline. Am J Kidney Dis 2015 Nov;66(5):823-836.
- 22. Unruh ML, Larive B, Chertow GM, Eggers PW, Garg AX, Gassman J, et al; FHN Trials Group. Effects of 6-times-weekly versus 3-times-weekly hemodialysis on depressive symptoms and self-reported mental health: Frequent Hemodialysis Network (FHN) Trials. Am J Kidney Dis 2013 May;61(5):748-758.
- 23. Mazairac AH, Grooteman MP, Blankestijn PJ, Penne EL, van der Weerd NC, den Hoedt CH, et al; CONTRAST investigators. Differences in quality of life of hemodialysis patients between dialysis centers. Qual Life Res 2012 Mar;21(2):299-307.
- 24. Cruz MC, Andrade C, Urrutia M, Draibe S, Nogueira-Martins LA, Sesso RdeC. Quality of life in patients with chronic kidney disease. Clinics (Sao Paulo) 2011;66(6):991-995.
- 25. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 2009 Oct;62(10):e1-e34.
- 26. Collaboration C. The Nordic Cochrane Centre. Review Manager. 2014.
- 27. Suurmond R, van Rhee H, Hak T. Introduction, comparison, and validation of Meta-Essentials: A free and simple tool for meta-analysis. Res Synth Methods 2017 Dec;8(4):537-553.
- 28. Sterne JA, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019 Aug;366:l4898.
- 29. Cukor D, Ver Halen N, Asher DR, Coplan JD, Weedon J, Wyka KE, et al. Psychosocial intervention improves depression, quality of life, and fluid adherence in hemodialysis. J Am Soc Nephrol 2014 Jan;25(1):196-206.
- 36. Duarte PS, Miyazaki MC, Blay SL, Sesso R. Cognitive-behavioral group therapy is an effective treatment for major depression in hemodialysis patients. Kidney Int 2009 Aug;76(4):414-421.
- 30. Erdley-Kass SD, Kass DS, Gellis ZD, Bogner HA, Berger A, Perkins RM. Using problem-solving therapy to improve problem-solving orientation, problem-solving skills and quality of life in older hemodialysis patients. Clin Gerontol 2018 Oct-Dec;41(5):424-437.
- Ghadam MS, Poorgholami F, Badiyepeymaie Jahromi Z, Parandavar N, Kalani N, Rahmanian E. Effect of self-care education by face-to-face method on the quality of life in hemodialysis patients (relying on ferrans and powers questionnaire). Glob J Health Sci 2015 Oct;8(6):121-127.
- 32. Lii YC, Tsay SL, Wang TJ. Group intervention to improve quality of life in haemodialysis patients. J Clin Nurs 2007 Nov;16(11C):268-275.
- Moattari M, Ebrahimi M, Sharifi N, Rouzbeh J. The effect of empowerment on the self-efficacy, quality of life and clinical and laboratory indicators of patients treated with hemodialysis: a randomized controlled trial. Health Qual Life Outcomes 2012 Sep;10(1):115.
- Rodrigue JR, Mandelbrot DA, Pavlakis M. A psychological intervention to improve quality of life and reduce psychological distress in adults awaiting kidney transplantation. Nephrol Dial Transplant 2011 Feb;26(2):709-715.
- Tsay SL, Lee YC, Lee YC. Effects of an adaptation training programme for patients with end-stage renal disease. J Adv Nurs 2005 Apr;50(1):39-46.
- 37. DeOreo PB. Hemodialysis patient-assessed functional health status predicts continued survival, hospitalization, and dialysis-attendance compliance. Am J Kidney Dis 1997 Aug;30(2):204-212.
- 38. Lowrie EG, Curtin RB, LePain N, Schatell D. Medical outcomes study short form-36: a consistent and powerful predictor of morbidity and mortality in dialysis patients. Am J Kidney Dis 2003 Jun;41(6):1286-1292.
- 39. Ware J, Kosinski M, Keller S. Physical and mental health summary scales: a user’s manual. Boston, MA: The Health Institute. 1994.
- 40. Beck J. Cognitive Behavior Therapy: Basics and Beyond, 2nd Edn New York. NY: Guilford Press (Google Scholar). 2011.
- 41. Hides L, Samet S, Lubman DI. Cognitive behaviour therapy (CBT) for the treatment of co-occurring depression and substance use: current evidence and directions for future research. Drug Alcohol Rev 2010 Sep;29(5):508-517.
- 42. Lazarus RS, Folkman S. Stress, appraisal, and coping: Springer publishing company; 1984.
- 43. Mitchell AJ, Selmes T. Why don’t patients take their medicine? Reasons and solutions in psychiatry. Adv Psychiatr Treat 2007;13(5):336-346