Antimicrobial resistance is a worrying worldwide health issue due to the high cost of medical treatment and its potentially severe repercussions.1 It occurs when the microorganism has an adaptive response when exposed to antimicrobial treatment.1 One of the causes of emerging antimicrobial resistance is multidrug-resistant tuberculosis (MDR-TB). According to the World Health Organization (WHO) global report, there were 480 000 new cases of MDR-TB in 2014, with only 48% successfully treated and approximately caused 210 000 deaths.2 Indonesia, which was considered as one of 30 countries with a high burden of MDR-TB, showed that the estimation of new cases of MDR-TB in 2019 was 2.4%.3
MDR-TB is considered a significant obstacle in achieving efficacious treatment of TB.4 Some factors that contribute to rising MDR-TB cases include inadequate medical monitoring systems, poor compliance, and incorrect treatment which could change resistance patterns, as well as community-based transmission.4 Moreover, in terms of therapeutic effectiveness, the success rate of MDR-TB treatment in Indonesia was only 45%.3 The WHO recommends a therapy duration of 20 months, but the success rate of the recommended treatment is still relatively low and does not exceed 50%.5 The recommendations of second-line treatment based on WHO guidelines in 2011 are fluoroquinolone (FQ), ethionamide (ETH) or protionamide (PTH), and cycloserine or para-aminosalicylic acid, with the addition of pyrazinamide (PZA) for a total duration of 20 months.6 The duration of the treatment will affect the compliance of the patients, therefore influencing the entirety of the course.
In this situation, using the short-term regimen (STR) to face the MDR-TB crisis as an alternate method proves promising.7,8 The STR could effectively reduce the duration of drug administration. Furthermore, STR is summarized into three drug classes, including FQs (ofloxacin, gatifloxacin (GFX), moxifloxacin (MFX)), core drugs (kanamycin (KM) and prothionamide), and active companion drugs (clofazimine (CFZ) and first-line drugs such as isoniazid).9
The treatment of MDR-TB using STR is crucial and essential to explore, as it can increase the success rate of MDR-TB treatment. Since the invention of the Bangladesh regimen in 2010, which only required nine months of treatment, several studies have also been carried out to implement a similar regimen in MDR-TB management.9,10 Several new regimens have also been reported to compare the effectiveness with long-term regimens.11,12
Related to the various emerging STRs in the past decade, an update on the application of STR in the management of MDR-TB is needed to increase the success rate of treatment. Therefore, this systematic review aims to explore the effectiveness and safety of various STR in treating MDR-TB. In the end, this systematic study is expected to contribute to more effective and safer treatment of MDR-TB in the community in the future.
Methods
This systematic review was constructed according to the rules of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA), which evaluated the effectiveness and safety of STR as an MDR-TB treatment. The writing of this report reviewed the effectiveness and safety of STR by comparing population, intervention, control, />and outcome (PICO) data. The population are patients diagnosed with MDR-TB; the intervention is STR, which is defined as the administration of several drug combinations and FQ options for 6–12 months. The control is the recommended therapy regimens standards published by WHO in 2011, and lastly, the outcome is to determine the effectiveness and adverse effects of STR.
Data sources were traced through several search engines, including ScienceDirect, PubMed, Ovid-MEDLINE, Cochrane, and Clinicaltrials.gov. Article tracing was done to identify studies and research published in medical journals in the last 10 years from January 2009 to December 2019, which focused on studies related to MDR-TB and management. The keywords were arranged based on PICO by utilizing Boolean searching and truncation to expand the area of inquiry, consisting of ‘multidrug-resistant tuberculosis’ or ‘MDR-TB’ and ‘short regimen’ or ‘short-term regimen’ or ‘short course regimen’. The search limitations applied through search engines included the type of article, the search period, and the year of the published article.
The inclusion criteria were: 1) patients in all age groups diagnosed with MDR-TB; 2) the studies which included STR with a duration of therapy of 6–12 months; 3) the studies which included the effectiveness and adverse effects of STR; 4) clinical studies which were published between January 2009 and December 2019; and 5) full-text articles published in English. Meanwhile, the exclusion criteria were the studies that did not meet the inclusion criteria, systematic reviews, and other meta-analysis articles. The specific keywords were used to generate chosen articles based on abstracts and full text. The selection of data sources referred to the inclusion criteria that were previously determined. After that, all abstracts and full texts were downloaded and evaluated. All complete texts that met the inclusion criteria were read independently by the authors and evaluated to formulate a systematic review [Figure 1].
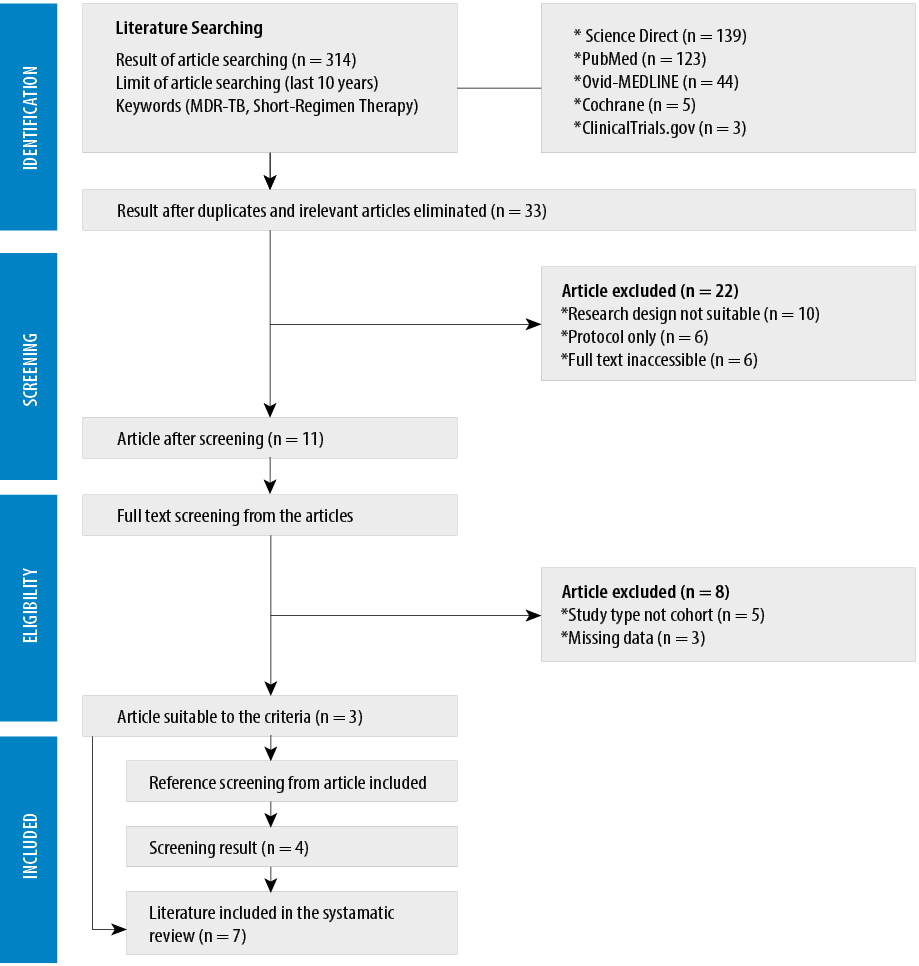
Figure 1: The Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram.
Results
We first found a total of 314 studies. Furthermore, there were also four additional studies included that were filtered from the reference list of articles used. After excluding irrelevant articles, 33 studies were found. The remaining 11 studies were then analyzed based on exclusion criteria, such as the study design and the completeness of the data.
After applying the inclusion criteria, seven studies published between 2010–2019 were obtained. We analyzed the risk of bias using the Newcastle-Ottawa scale [Appendix 1]. Every study was a prospective cohort taken from different countries, such as Bangladesh, Nigeria, Cameroon, nine countries in Africa, and China.5,9–15
The data used in those studies came from clinical trials performed between 1997 and 2016: two studies with a duration of two years,5,10 one study with a duration of three years,14 one study spanning four years,12 two studies spanning six years,11,13 and one study spanning 10 years.9 Of the seven studies, two studies compared STR with long-term therapy (LTR),11,12 while the other five studies only investigated STR.5,9,10,13,14 The total subjects analyzed in this systematic review were 2157 patients aged 12 to 80 years old. The TB drug sensitivity analyzed in early diagnosis of the patient for each study was the resistance to isoniazid (INH) and rifampin (RMP) and met the definition of MDR-TB.5,9–14 Two other studies also explained the resistance to FQs.5,13 HIV status was analyzed in three studies, whereas the four other studies did not analyze HIV status due to the limitation of studies.9,11–13 The characteristics of the studies are shown in Table 1.
Table 1: Characteristics of previous studies.
|
Van Deun et al,9 2010 |
Prospective cohort study |
1997–2007
(10 years) |
Bangladesh |
206 |
33.8
(24–55) |
+ |
+ |
- |
- |
|
Aung et al,13 2014 |
Prospective cohort study |
2005–2011
(6 years) |
Bangladesh |
515 |
44
(12–76) |
+ |
+ |
+ |
- |
|
Piubello et al,10 2014 |
Prospective cohort study |
2008–2010
(2 years) |
Niger |
65 |
31
(16–66) |
+ |
+ |
- |
1.7%
(1/65) |
|
Kuaban et al,14 2015 |
Prospective cohort study |
2008–2011
(3 years) |
Cameroon |
150 |
42.5
(17–68) |
+ |
+ |
- |
20%
(30/150) |
|
Trébucq et al,5 2018 |
Prospective collaborative observational study |
2013–2015
(2 years) |
9 countries in Africa |
1006 |
34
(18–80) |
+ |
+ |
+ |
19.9% (200/1006) |
|
Yan et al,11 2018 |
Prospective, multicenter, cohort study |
2009–2015
(6 years) |
China |
80;
61 STR
19 LTR |
41.5
(18–65) |
+ |
+ |
- |
- |
INH: isoniazid; RMP: rifampin; FQ: fluoroquinolone; STR: short-term regimen; LTR: long-term therapy.
Analysis of STR composition
As the incidence of MDR-TB rises, the TB drugs that used to be divided into two groups are now divided into five major groups. Group 1 consists of first-line oral drugs, such as rifabutin (RFB), ethambutol (EMB), and INH.15 The type of INH used is high dose INH (INHh), with the considerations of being effective in patients with low-level resistance toward INH and is able to eradicate bacteria strain which also resistant to PTH. It was reported that some individuals with low-level resistance to INH have resistance to PTH.5 EMB is still used in the STR because of its effectiveness.14 RFB is also used as a STR choice because it has a higher affinity to bacterial RNA polymerase compared to RMP.11 In this systematic review, RFB was used in one regimen11, INHh in five regimens,5,9,10,13,14 and EMB in six regimens.5,9–11,13,14
Group 2 consists of injectable agents such as KM.15 KM is often used in the STR because of its efficacy and affordability.16 In this systematic review, KM was used in five regimens.5,9,10,13,14
Group 3 consists of FQ and PZA.15 Some FQ options that were used in MDR-TB STR include GFX, MFX, and levofloxacin (LFX). The consideration of choosing FQ is due to the effectiveness and possible future resistance.17 GFX was used in four regimens,9,10,13,14 while MFX is in two regimens,5,11 and LFX in one regimen.12 Furthermore, PZA is also used as a sterilizing drug with comparable efficacy to RMP in increasing the effectiveness of FQ.18 PZA was used in every regimen.5,9–14
Group 4 consists of second-line TB drugs, including PTH, CS, and para-aminosalicylic acid, such as pasiniazid (PSD).15 PTH is a bactericidal agent used in STR due to its high efficacy.19 CS has been used as an anti-TB agent since 1950 but lost favor after discovering better options, such as rifampicin.20 PSD, a combination drug made from p-aminosalicylic acid and INH, is chosen for MDR-TB treatment because > 80% of patients with resistance to INH still responded to PSD.21 In this systematic review, PSD was used in one regimen,11 CS in one regimen,12 and PTH in six regimens.5,9,10,12–14
Group 5 consists of drugs whose efficacy had not been proven in MDR-TB, such as CFZ.15 The CFZ was used as an option in the STR because of its high effectiveness and tolerability as companion drugs.20 In this systematic review, CFZ is used in six regimens.5,9,10,12–14
Overall, the STR in this systematic review consists of at least one anti-TB drug in group 1, one in group 2, PZA and one group of FQ in group 3, one in group 4, and CFZ in group 5. Some reported regimens have exceptions, such as one regimen not using anti-TB drugs in group 111 and one regimen not using anti-TB drugs in group 5.12 The regimens recommended by the WHO in 2011 consisted of only three groups, including one anti-TB drug in group 2, PZA and one group of FQ in group 3, and one in group 4. The effectiveness and safety of each regimen will be explained in the following subsections.
The effectiveness of STR in terms of success rate and duration of STR administration
Generally, STR have a better therapeutic effect and shorter duration than the 2011 WHO regimen for MDR-TB, with treatment success rates > 50% for each study.6 Four studies had success rates > 80%9,10,13,14 and three other studies < 80% [Figure 2].5,11,12 One study that reported a success rate of < 80% was due to the high mortality rate, which was unrelated to the effectiveness of STR (such as starvation and HIV infection). In hindsight, the success rate of therapy in patients who survived was quite high at 88.9%.5
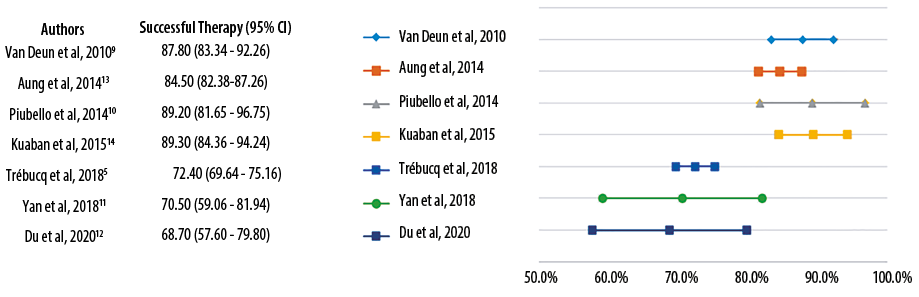
Figure 2: The comparison of treatment success rates of short-term regimen.
Likewise, two other studies with therapeutic success rates of < 80% have a smaller pool of samples which caused a wide range of confidence intervals. However, they have shown better therapeutic success rates than the LTR, although with a fairly narrow difference (STR 70.5% and 68.7%, LTR 63.1% and 64.7%).11,12 These two studies used regimens that were slightly different from others. One study, with a 70.5% success rate, used the STR with the shortest duration (five months) with the addition of PSD and RFB instead of INH and RMP in the TB without drug resistance.11 The other study with a success rate of 68.7% used STR for 12 months with the addition of CS.12
According to a study conducted by Li et al,20 in 2019, the single-drug administration of CS had a good outcome and proved to be safe with fewer adverse reactions compared to other anti-TB drugs.20 RFB and PSD were also reported to have good efficacies, and the administration could reduce the risk of different MDR-TB strain transmission.21,22 However, the concept of TB treatment is directed at the regimen’s effectiveness and not in the form of a single drug administration.23 Even though indivually RFB, PSD, and CS have good potency, more evidence is needed of those drugs in one regimen to evaluate their efficacy in MDR-TB. In addition, there is still a lack of study regarding the efficacy of administering a similar regimen compared to the two studies mentioned previously.
Three studies used the same regimen consisting of KM, INH, CFZ, EMB, PTH, PZA, GFX in the intensive phase, and CFZ, EMB, PZA, and GFX in the continuation phase [Appendix 2].9,10,13 These studies reported a therapeutic success rate of > 80%.9,10,13 The duration of the three studies were slightly varied; two studies used four months intensive phase and five months intensive phase,9,13 with similar success rates (87.8% and 84.5%), and the relapse rate after two years was quite low (0.5% and 0.8%, respectively).9,13 In the other two studies, the continuation phase had a longer duration compared to the three studies with a span of eight months.10,14 This addition of three months gave a therapeutic success rate of 89.2%, and no relapse was reported after two years of follow-up.10 Another study revealed the administration of similar regimens with the addition of eight months of PTH in the continuation phase, with a success rate of 89.3% and without relapse after two years.14
Three studies revealed the effectiveness of STR in patients with HIV comorbidity.5,10,14 The success rate of < 80% was found in one study, likely due to high mortality in HIV patients. However, if the success rate of therapy was calculated from surviving HIV patients, the success rate reached 88.4%.5 The other four studies did not include patients
with HIV.9–14
Fluoroquinolone option in STR
The use of FQ is essential in composing STRs for MDR-TB. The majority of studies used GFX as an FQ option in STR.9,10,13,14 One study replaced the FQ from GFX to MFX and had a < 80% success rate.5 This might occur due to the fact that in two years of therapy, 1.4% of total patients had a high-level resistance to FQ.5 STR using LFX also had a < 80% success rate. These results are supported by a study conducted by Van Deun et al.,17 in 2019, where the use of GFX had a higher effectiveness (97.5%) compared to LFX and MFX (95.5% and 94.7%) with a lower incidence of adverse effects. In addition, compared to GFX, patients given LFX and MFX therapy tended to form resistance to FQ, which were respectively 4.5 and 8.4 times higher than GFX.17
Resistance to FQ is an important aspect to consider in composing the STR. In one study, the FQ resistant group had a successful therapeutic rate (70.96%). However, if it was classified into two groups of low-level resistance and high-level resistance, the high-level resistance group had a lower success rate (46.67%).13 Similar results were also reported in another study, with a therapeutical success rate of 59.2% in the group with FQ resistance and 55.6% in the group with high-level resistance.5 A literature review by Trébucq et al,23 compared the speed of FQ resistance development by FQ with GFX with rifampicin. The resistance development speed of rifampicin is 1 per 1000 patients, whereas in FQ other than GFX the resistance development can reach up to 10–20 per 1000 patients after six months.23 This dangerous speed of resistance may pose a serious threat to public health.23
The latest Indonesian recommendations for MDR-TB published in 2016 still use the MFX option as FQ.22 The consideration to replace the FQ option from MFX to GFX is needed to increase the effectiveness of MDR-TB therapy in the future. This substitution may require the help of the WHO because this drug still cannot be purchased in some countries. Therefore, it needs to be included in the WHO Model List of Essential Medicines.23
Unsuccessful treatment and relapse in STR
The number of unsuccessful, failed, and default treatments, as well as the subjects who did not survive, are related to the success rate of each STR used [Appendix 3]. A study reported that the mortality rate was the main cause of therapy failure (9.2%), but this was mainly due to low BMI, old age, extensive pulmonary lesions, and HIV infection; and therefore, not affecting the effectiveness of the overall regimen.10 The relapse rates were reported in four studies after two years of follow-up, with two studies reporting no relapse10,14 and two other reported relapse rates < 1%.9,13
The STR safety in terms of side effects
Overall, the four most reported side effects were gastrointestinal problems, ototoxicity, dysglycemia, and liver problems [Table 2]. Five studies reported mostly gastrointestinal side effects (21.4%, 21.6%, 36.9%, 57.1%, and 3.0%).5,9,10,12,13 This side effect was probably caused by the use of PTH in the continuation phase.9,10 Another side effect was ototoxicity in five studies (6.3%, 1.4%, 20.0%, 16.0%, and 44.3%) which was caused by KM.5,9,10,13,14 Dysglycemia occurred in three studies (3.9%, 1.6%, 9.2%) due to GFX.9,10,13 Side effects involving the liver were shown in three studies (0.7%,
48.8%, 16.4%).5,12,14
Table 2: Side effects related to short-term regimen.
|
Ototoxicity |
6.3%
(13/206) |
1.4%
(7/515) |
20.0%
(13/65) |
16.0%
(24/150) |
44.3%
(446/1006) |
- |
- |
- |
|
Gastrointestinal |
21.4% (44/206) |
21.6%
(111/515) |
36.9%
(24/65) |
- |
57.1%
(574/1006) |
- |
3.0%
(2/67) |
2.9%
(2/68) |
|
Psychiatry |
0.5%
(1/206) |
- |
6.2%
(4/65) |
- |
- |
- |
3.0%
(2/67) |
1.5%
(1/68) |
|
Dysglycemia |
3.9%
(8/206) |
1.6%
(8/515) |
9.2%
(6/65) |
- |
- |
- |
- |
- |
|
Renal |
- |
- |
- |
- |
15.7%
(158/1006) |
- |
7.5%
(5/67) |
4.4%
(3/68) |
|
Liver |
- |
- |
- |
0.7%
(1/150) |
48.8%
(491/1006) |
- |
16.4%
(11/67) |
19.1%
(13/68) |
|
Skin |
- |
- |
3.1%
(2/65) |
- |
- |
- |
10.4%
(7/67) |
0.0%
(0/68) |
|
Ophthalmology |
- |
- |
3.1%
(2/65) |
- |
- |
- |
- |
- |
|
Musculoskeletal |
1.0%
(2/206) |
- |
6.2%
(4/65) |
- |
18.2%
(183/1006) |
- |
- |
- |
|
Neurology |
3.9%
(8/206) |
- |
6.2%
(4/65) |
0.7%
(1/150) |
26.9%
(271/1006) |
- |
- |
- |
LTR: long-term regimen.
Six studies reported a level of side effects of < 30%.5,9,10,12–14 In one study with a side effect level of > 30%, it was the result of a calculation that factored mild to severe symptoms (from grade 1 to grade 5). However, the study explained that cessation of the treatment for the patient due to the side effects was not needed. Overall, there was only one study that had to stop therapy because of the side effects in two patients.11 The other five studies reported no discontinuation of therapy due to the side effects found. However, some adjustments related to dosage and drug use are still made in several studies.5,9–11,13,14
Conclusion
STR provides better benefits in MDR-TB treatment, particularly in its effectiveness and the short duration of therapy. STR is relatively safe and has minimal side effects that can be tolerated in most patients. The STR combination analyzed in this systematic review consisted of at least one anti-TB drug in group one, one in group two, PZA and one group of FQ in group 3, one in group 4, and CFZ in group 5. The suggested option for FQ is GFX, considering the aspects of effectiveness, safety, and resistance development to FQ that might occur. The most effective regimen according to studies analyzed in this review is KM-INH-CFZ-EMB-PTH-PZA-GFX in the intensive phase for four months and CFZ-EMB-PZA-GFX-PTH in the continuation phase for eight months. This systematic review has a limitation. There was no heterogeneity analysis of each study used. These limitations could open the opportunity to compile other meta-analyses to assess the heterogeneity of the data and the formation of quantitative conclusions in the future. Further research into the success rate of several new STR is needed to assess the effectiveness in various other settings. It is also possible to perform a study that could compare the effectiveness of the regimen composition in each anti-TB group to produce a safer STR, or another systematic review that evaluates randomized control trial studies covering the same topic. The development of STR management for MDR-TB is not infallible yet. However, with evidence in the form of further research on the STR management methods, an ideal treatment for MDR-TB might be discovered.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
references
- 1. World Health Organization. Antimicrobial resistance. 2021 [cited 2021 December 17]. Available from: https://www.who.int/en/news-room/fact- sheets/detail/antimicrobial-resistance.
- 2. World Health Organization. Global tuberculosis report. Geneva; 2014.
- 3. World Health Organization. Global tuberculosis report. Geneva; 2020.
- 4. Seung KJ, Keshavjee S, Rich ML. Multidrug-resistant tuberculosis and extensively drug-resistant tuberculosis. Cold Spring Harb Perspect Med 2015 Apr;5(9):a017863.
- 5. Trébucq A, Schwoebel V, Kashongwe Z, Bakayoko A, Kuaban C, Noeske J, et al. Treatment outcome with a short multidrug-resistant tuberculosis regimen in nine African countries. Int J Tuberc Lung Dis 2018 Jan;22(1):17-25.
- 6. World Health Organization. WHO treatment guidelines for drug-resistant tuberculosis 2016. Geneva; 2016.
- 7. World Health Organization. Guidelines for the programmatic management of drug-resistance tuberculosis. Geneva; 2011.
- 8. Szumowski JD, Lynch JB. Profile of delamanid for the treatment of multidrug-resistant tuberculosis. Drug Des Devel Ther 2015 Jan;9:677-682.
- 9. Van Deun A, Maug AK, Salim MA, Das PK, Sarker MR, Daru P, et al. Short, highly effective, and inexpensive standardized treatment of multidrug-resistant tuberculosis. Am J Respir Crit Care Med 2010 Sep;182(5):684-692.
- 10. Piubello A, Harouna SH, Souleymane MB, Boukary I, Morou S, Daouda M, et al. High cure rate with standardised short-course multidrug-resistant tuberculosis treatment in Niger: no relapses. Int J Tuberc Lung Dis 2014 Oct;18(10):1188-1194.
- 11. Yan L, Kan X, Zhu L, Xu K, Yin J, Jie L, et al. Short-course regimen for subsequent treatment of pulmonary tuberculosis: a prospective, randomized, controlled multicenter clinical trial in China. Clin Ther 2018 Mar;40(3):440-449.
- 12. Du Y, Qiu C, Chen X, Wang J, Jing W, Pan H, et al. Treatment outcome of a shorter regimen containing clofazimine for multidrug-resistant tuberculosis: a randomized control trial in China. Clin Infect Dis 2020;71(4):1047-1054.
- 13. Aung KJ, Van Deun A, Declercq E, Sarker MR, Das PK, Hossain MA, et al. Successful ‘9-month Bangladesh regimen’ for multidrug-resistant tuberculosis among over 500 consecutive patients. Int J Tuberc Lung Dis 2014 Oct;18(10):1180-1187.
- 14. Kuaban C, Noeske J, Rieder HL, Aït-Khaled N, Abena Foe JL, Trébucq A. High effectiveness of a 12-month regimen for MDR-TB patients in Cameroon. Int J Tuberc Lung Dis 2015 May;19(5):517-524.
- 15. Shim TS, Jo KW. Medical treatment of pulmonary multidrug-resistant tuberculosis. Infect Chemother 2013 Dec;45(4):367-374.
- 16. Sirgel FA, Warren RM, Böttger EC, Klopper M, Victor TC, van Helden PD. The rationale for using rifabutin in the treatment of MDR and XDR tuberculosis outbreaks. PLoS One 2013;8(3):e59414.
- 17. Van Deun A, Decroo T, Kuaban C, Noeske J, Piubello A, Aung KJ, et al. Gatifloxacin is superior to levofloxacin and moxifloxacin in shorter treatment regimens for multidrug-resistant TB. Int J Tuberc Lung Dis 2019 Sep;23(9):965-971.
- 18. Chang KC, Leung CC, Yew WW, Leung EC, Leung WM, Tam CM, et al. Pyrazinamide may improve fluoroquinolone-based treatment of multidrug-resistant tuberculosis. Antimicrob Agents Chemother 2012 Nov;56(11):5465-5475.
- 19. Scardigli A, Caminero JA, Sotgiu G, Centis R, D’Ambrosio L, Migliori GB. Efficacy and tolerability of ethionamide versus prothionamide: a systematic review. Eur Respir J 2016 Sep;48(3):946-952.
- 20. Li Y, Wang F, Wu L, Zhu M, He G, Chen X, et al. Cycloserine for treatment of multidrug-resistant tuberculosis: a retrospective cohort study in China. Infect Drug Resist 2019 Mar;12:721-731.
- 21. Yan L. Drug resistance analysis of pasiniazid against isoniazid-resistant, parasal-resistant, isoniazid and parasal-resitant, and multidrug-resistant. Med Chem (Los Angeles) 2015;5:261-262.
- 22. Kementerian Kesehatan Republik Indonesia. Peraturan Menteri Kesehatan Republik Indonesia Nomor 67 Tahun 2016 tentang Penanggulangan Tuberkulosis. Kementerian Kesehatan Republik Indonesia. 2016.
- 23. Trébucq A, Decroo T, Van Deun A, Piubello A, Chiang C-Y, Koura KG, et al. Short-course regimen for multidrug-resistant tuberculosis: a decade of evidence. J Clin Med 2019 Dec;9(1):55.