An estimated 1.3 million men were diagnosed with prostate cancer (PCa), making it the fourth most common cancer worldwide in 2018.1 More than 350 000 men died from PCa during the same year.1 The incidence of PCa is lower in Arabs compared to western countries.2 The lower incidence may be partly attributed to lack of screening programs and the lower prostate-specific antigen (PSA) levels due to smaller prostate gland size amongst Arabs.2–4 However, the incidence of PCa in Asian countries, including Oman, has been gradually increasing over the years. PCa was the sixth most common cancer in Oman in 2015.5–7 The incidence is less compared to Bahrain and Kuwait, but higher than the UAE and Saudi Arabia.5,8
Medical or surgical castration remains the gold standard treatment for metastatic prostate cancer (mPCa). The disease escapes the lower testosterone levels for most men, and the cancer develops resistance to androgen deprivation, known as castrate-resistant PCa (CRPC).9,10 Docetaxel was the first agent to improve median overall survival (OS) and the quality of life (QoL) in mCRPC.9 Better understanding of the disease biology led to the introduction of newer agents. Various trials have established the efficacy of agents blocking androgen synthesis in improving the outcome of mCRPC. Together with docetaxel, these agents are now also used to treat hormone-sensitive mPCa (mHSPC).9,10 Docetaxel, abiraterone, and enzalutamide have been approved for the treatment of mHSPC while the same agents along with radium-223, sipuleucel-T, and cabazitaxel have been approved for the treatment of mCRPC.9,10
Differences in PCa incidence and associated mortality have been well recognized according to race and ethnicity despite living in the same or different countries. These differences have been attributed to genetic and environmental factors and access to health care and screening programs.1,4 Substantial data are available from other parts of the globe about survival rates of mHSPC or mCRPC, but there is a scarcity of information from the Middle-Eastern region.
Various studies from the region have reported the epidemiology of PCa, but limited data is available regarding the outcome of the PCa in Arabs.2,3 We report the presenting features, treatment, and outcome of men diagnosed with mPCa as well as factors affecting survival in Omani men. To the best of our knowledge, this is the first study reported from this region describing the survival outcomes for men with mPCa.
Methods
Consecutive patients diagnosed with mPCa at the Sultan Qaboos University Hospital (SQUH) between January 2006 and December 2017 were included in this study. SQUH is one of the two major hospitals in Muscat, providing cancer care to patients from all over Oman. The majority of the patients were diagnosed and treated at SQUH. In cases where the diagnosis was established elsewhere, the tissue blocks were reviewed at SQUH.
Electronic patient records (EPRs) were reviewed for demographic characters (age, comorbid conditions, and use of medicines for those conditions), clinicopatholgical features at presentation (PSA, Gleason score, clinical stage, organs involved, patient’s performance status [PS]), the treatment received (first line and subsequent lines), and the survival until either the last date of follow-up or the date of death. Nadir PSA levels, as well as time to nadir PSA and time to PSA decline by 50% were also checked.
We used the American Joint Committee on Cancer staging manual (8th edition) to stage the disease.10 Nadir PSA was the lowest PSA level documented on first-line treatment for mPCa, while time to nadir PSA was the time from the start of the treatment to the time to reach nadir PSA level.11 Patients with continuously rising PSA and testosterone levels below 1.7 nmol/L (0.50 ng/dL) were considered to have mCRPC.10 First-line and subsequent treatment offered for mPCa were recorded. Patients diagnosed to have mPCa after or before the date of study period, not treated at SQUH, those who lost to follow-up for more than two years, or had incomplete data on EPR, and the patients with localized PCa were excluded from the analysis.
We used the Kaplan-Meier method to estimate progression-free survival (PFS) and OS. PFS was defined as the time from the date of diagnosis of mPCa until disease progression. OS was defined as the time from diagnosis to death or 30 April 2019, while OS2 was defined as the survival from the date of mCRPC to death or 30 April 2019. The chi-square test was used for dichotomous variables, while Log-rank test and Cox regression analysis were used for time to event. A p-value of < 0.05 was considered significant. We used SPSS Statistics (IBM Corp. Released 2011. IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp.) for statistical analysis. Institutional medical research ethics committee approval was sought and given.
Results
A total of 239 patients were diagnosed with PCa during the study period. Out of those, 62 had mPCa and met the inclusion criteria. This study reported on the presenting features and outcomes of the 62 patients.
The median age of the patients was 71 years (range = 57–92). The vast majority of patients had at least one or more comorbidities (87.1%), hypertension being the most common (58.1%). Of all the patients, 25 (40.3%) took a statin, and 12 (19.4%) were on metformin for diabetes mellitus.
Trans-rectal ultrasound-guided biopsy was the most common diagnostic method (61.3%). More than half of the patients (61.3%) had a Gleason score of ≥ 8 at diagnosis, and 56.8% had Gleason score of 5 as primary or secondary pattern. Median PSA at the time of diagnosis was 100.0 ng/dL (range 3.0–4508.0), 50.0% of patients had PSA level of > 100.0 ng/dL at diagnosis. Staging studies revealed that 60 (96.8%) patients had stage 4b disease. Bones were the most frequent site of metastases (90.3%), 40 (64.5%) patients had > 10 lesions as seen on the bone scan [Table 1].
Table 1: Clinicopathological features of all patients.
|
Median age, years |
71 |
|
Comorbid conditions |
|
None |
8 (12.9) |
|
Hypertension |
36 (58.1) |
|
Dyslipidemia |
22 (35.5) |
|
Diabetes |
19 (30.6) |
|
Coronary artery disease |
12 (19.4) |
|
Statin use |
25 (40.3) |
|
Metformin |
12 (19.4) |
|
Diagnostic method |
|
TRUS* |
38 (61.3) |
|
TURP‡ |
15 (24.2) |
|
Other Biopsy |
7 (11.3) |
|
PSA only |
2 (3.2) |
|
Gleason score |
|
≤ 6 |
6 (9.7) |
|
7 |
11 (17.7) |
|
8 |
14 (22.6) |
|
9 |
19 (30.6) |
|
10 |
5 (8.1) |
|
Missing |
7 (11.3) |
|
PSA level |
|
< 10 |
5 (8.1) |
|
10–20 |
2 (3.2) |
|
21–50 |
12 (19.4) |
|
51–100 |
10 (16.1) |
|
> 100 |
31 (50.0) |
|
Missing |
2 (3.2) |
|
Site of metastasis |
|
Bone |
56 (90.3) |
|
Distant lymph nodes |
19 (30.6) |
|
Lungs |
6 (9.7) |
|
Liver |
3 (4.8) |
|
Number of bone metastases |
|
< 4 |
10 (16.1) |
|
4–10 |
6 (9.7) |
|
> 10 |
40 (64.5) |
TRUS: Transrectal ultrasound-guided biopsy; TURP: transurethral resection of the prostate; PSA: prostate-specific antigen.
Of the 62 patients, 27 (43.5%) received androgen deprivation therapy (ADT) (luteinizing hormone-releasing hormone analogue or anti-testosterone alone), while 24 patients (38.7%) were treated with combined androgen blockade (CAB). Only six (9.7%) patients received upfront docetaxel along with the ADT. Median time to nadir PSA was six months (range = 0–44 months), with a median nadir PSA level of 0.55 ng/dL (range = 0.01–1623.00). Median time to 50.0% drop in PSA was two months (range = 2–39). A minimum testosterone level of 0.7 nmol/L was achieved in 79.0% of patients. Minimum testosterone levels were achieved within six months in 25.8% of patients [Table 2].
Table 2: First-line treatment offered to all patients.
|
First-line treatment |
|
ADT* only |
27 (43.5) |
|
Combined androgen blockade |
24 (38.7) |
|
ADT and docetaxel |
6 (9.7) |
|
Surgical castration only |
2 (3.2) |
|
Radical prostatectomy and ADT |
2 (3.2) |
|
ADT and IMRT† |
1 (1.6) |
|
Time to nadir PSA, months |
|
< 6 |
30 (48.4) |
|
> 6 |
30 (48.4) |
|
Missing |
2 (3.2) |
|
Minimum testosterone level |
|
< 0.7 |
49 (79.0) |
|
0.7–1.7 |
2 (3.2) |
|
Missing |
11 (17.7) |
|
Time to minimum testosterone level, months |
|
< 6 |
16 (25.8) |
|
> 6 |
35 (56.5) |
ADT: androgen deprivation therapy; IMRT: intensity modulated radiation therapy; PSA: prostate-specific antigen.
The vast majority of patients (n = 40, 64.5%) developed mCRPC, slightly more than a quarter of patients (n = 17, 27.4%) remained on their first line treatment, while in one patient neuroendocrine transformation was documented, whose disease continued to progress causing painful lymphadenopathy in the groin with a PSA of 0.1. Biopsy of lymph node mass confirmed the diagnosis of neuroendocrine transformation of PCa [Table 3]. PSA level was > 20 ng/dL in 28 (68.3%) patients who developed mCRPC. Radiological progression was documented in 38 (92.7%) patients, while in the remaining two patients, a high PSA level with a low testosterone level was considered an indication of disease progression. Bone was the most common site of disease progression 29 (70.7%). At the time of confirmed mCRPC, 65.2% of patients had a performance status of 0–1. Most patients (n = 30, 75.0%) who developed mCRPC were treated with systemic cancer therapy. Abiraterone and docetaxel were the two most common agents used [Table 3]. Of the patients treated with either docetaxel or abiraterone, one-third of patients (n = 11, 36.7%) required dose reduction at some point during the treatment trajectory. PSA dropped by > 50.0% in 13 (44.8%) patients within three months of starting the treatment. Disease progression was documented in 25 (86.2%) patients. Abiraterone was the most commonly used treatment option as the second-line treatment for mCRPC [Table 3].
Table 3: Characteristics, treatment offered, and outcome of patients with progressive prostate cancer (n = 41).
|
Progressed to mCRPC* |
40 (64.5) |
|
Hormone sensitive |
17 (27.4) |
|
Transformation to neuroendocrine tumor |
1 (1.6) |
|
Missing |
4 (6.5) |
|
PSA level at mCRPC |
|
|
≤ 20 |
12 (29.3) |
|
> 20 |
28 (68.3) |
|
Missing |
1 (2.4) |
|
Radiological progression |
|
|
Yes |
38 (92.7) |
|
No |
2 (4.9) |
|
Missing |
1 (2.4) |
|
Site of radiological progression |
|
|
Bone only |
29 (70.7) |
|
Lymph nodes only |
3 (7.3) |
|
Viscera and lymph nodes |
3 (7.3) |
|
Viscera only |
2 (4.8) |
|
Bone and lymph node |
1 (2.4) |
|
Bone, viscus, and lymph node |
1 (2.4) |
|
Missing |
2 (4.8) |
|
Performance status at mCRPC |
|
|
0 |
6 (14.6) |
|
1 |
21 (51.2) |
|
2 |
5 (12.2) |
|
3 |
7 (17.1) |
|
4 |
3 (7.3) |
|
First-line therapy for mCRPC |
|
|
Abiraterone with prednisolone |
12 (29.2) |
|
Docetaxel ± prednisolone |
10 (24.4) |
|
Enzalutamide |
6 (14.6) |
|
Best supportive care |
9 (22.0) |
|
Cabazitaxel† |
1 (2.4) |
|
Etoposide and carboplatin |
1 (2.4) |
|
Patient declined therapy |
1 (2.4) |
|
Lost to follow-up |
1 (2.4) |
|
Adverse events |
|
|
None |
16 (55.2) |
|
Fatigue |
8 (27.6) |
|
Neutropenia |
2 (6.9) |
|
Anemia |
1 (3.4) |
|
Volume overload |
1 (3.4) |
|
PSA response to treatment |
|
|
PSA dropped by > 50% in three months |
13 (44.8) |
|
PSA dropped by > 50% in six months |
15 (51.7) |
|
PSA increased |
7 (24.1) |
|
Disease progression |
|
|
Radiological and PSA progression |
19 (65.5) |
|
PSA progression |
5 (17.2) |
|
Radiological progression |
1 (3.4) |
|
Missing |
4 (13.8) |
|
Second-line treatment (n = 22) |
|
|
Abiraterone with prednisolone |
9 (40.9) |
|
Docetaxel ± prednisolone |
3 (13.6) |
|
Enzalutamide |
3 (13.6) |
|
Cabazitaxel |
1 (4.5) |
|
Irinotecan |
1 (4.5) |
|
Best supportive care |
5 (22.7) |
|
Bone modifying agents |
|
|
Zoledronic acid |
22 (53.7) |
|
Denosumab |
9 (22.0) |
|
Zoledronic acid followed by denosumab |
4 (9.8) |
|
Alendronate |
1 (2.4) |
|
Skeletal events |
|
|
Bone pain requiring radiotherapy |
12 (29.3) |
|
Bone pain requiring opiates |
7 (17.1) |
mCRPC: metastatic castration-resistant prostate cancer; PSA: prostate-specific antigen. Patient developed mCRPC soon after docetaxel.
Bone modifying agents were used in 36 patients, zoledronic acid being the commonest agent (n = 22, 53.7%). Twenty (48.8%) patients had skeletal-related event, worsening bony pain requiring palliative radiotherapy being the commonest (n = 12, 29.3%) [Table 3].
After a median follow-up of 34.5 months (range = 1.0–93.0), four (6.5%) are in complete remission, 16 (25.8%) were still receiving treatment, and 20 (32.3%) died of disease progression; 13 (21.0%) died of a cause unrelated to disease, or its treatment, one (1.6%) died of treatment associated toxicity, one received best supportive care measures, while seven patients (11.3%) were lost to follow-up. Median PFS was 17.0 (range = 4.0–91.0) months [Figure 1]. Age, bones metastases, number of bone metastases, time to nadir PSA, nadir PSA level, testosterone level of < 0.7 nmol/L, and type of first-line treatment significantly affected the PFS on univariate analysis [Table 4]. None of the factors significantly affected PFS on multivariate analysis.
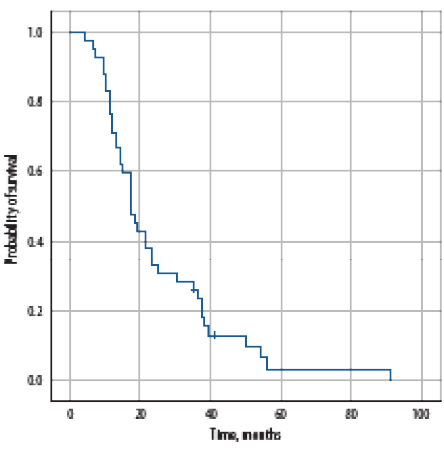
Figure 1: Progression-free survival of all patients with metastatic prostate cancer at the time of diagnosis.
At a median follow-up of 34.5 months (range = 1.0–93.0), the median OS was 43 months (range = 1–93, 95% confidence interval: 95%, 33.5–52.5) [Figure 2a]. Age, number of comorbidities, hypertension, statins use, Gleason score, number of bone metastases [Figure 2b], liver metastasis, PSA level at diagnosis, time to nadir PSA, nadir PSA level, testosterone level [Figure 2c], complication with first-line treatment, PFS, PSA levels at the time of mCRPC, visceral metastasis, PS at the time of disease progression, treatment duration for first-line treatment for mCRPC, use of bone modifying agents, and occurrence of skeletal-related event significantly affected the OS-1 on univariate analysis [Table 4]. None of the factors significantly affected PFS on multivariate analysis.
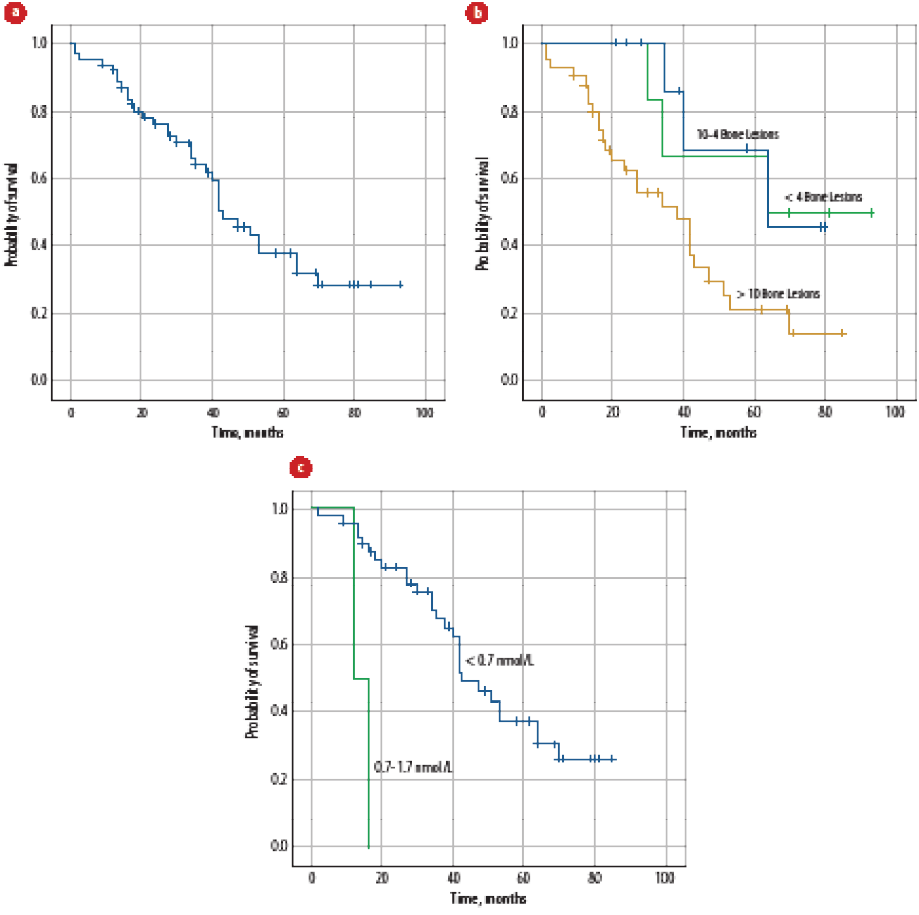
Figure 2: (a) Overall survival of all patients with metastatic prostate cancer (mPCa) at diagnosis. (b) Overall survival of all patients with mPCa at the time of diagnosis according to the number of bone metastasis. (c) Overall survival of all patients with mPCa at the time of diagnosis according to drop in testosterone levels.
Table 4: Univariate analysis for progression-free survival (PFS) and overall survival (OS) 1 for all patients with metastatic prostate cancer (n = 62).*
|
Diagnostic method |
|
TRUS |
19.0 |
2.6 |
13.8–24.1 |
0.040 |
53.0 |
7.1 |
39.0–67.0 |
< 0.001 |
|
TURP |
17.0 |
3.5 |
10.0–23.9 |
|
35.0 |
3.9 |
27.3–42.6 |
|
|
PSA |
10.0 |
- |
- |
|
1.0 |
- |
- |
|
|
Other biopsy |
11.0 |
2.1 |
6.7–15.2 |
|
42.0 |
15.8 |
11.0–73.0 |
|
|
Comorbidities |
|
|
|
|
|
|
|
|
|
None |
11.0 |
0.5 |
9.9–12.0 |
0.170 |
13.0 |
3.5 |
6.0–20.0 |
0.002 |
|
One |
17.0 |
13.8 |
0.0–44.1 |
|
64.0 |
17.2 |
30.1–97.8 |
|
|
Two |
19.0 |
2.8 |
13.3–24.6 |
|
47.0 |
5.8 |
35.5–58.4 |
|
|
Three |
18.0 |
4.4 |
9.2–26.7 |
|
42.0 |
8.7 |
25.0–59.0 |
|
|
Statin use |
|
Yes |
18.0 |
2.0 |
14.0–21.9 |
0.240 |
Not reached |
- |
- |
0.007 |
|
No |
14.0 |
2.5 |
9.1–18.8 |
|
40.0 |
4.2 |
31.7–48.2 |
|
|
Gleason score |
|
6 |
18.0 |
8.9 |
0.4–35.6 |
0.320 |
27.0 |
18.0 |
0.0–62.2 |
0.023 |
|
7 |
19.0 |
6.3 |
6.6–31.3 |
|
51.0 |
- |
- |
|
|
8 |
21.0 |
5.2 |
10.7–31.2 |
|
Not reached |
- |
- |
|
|
9 |
14.0 |
3.1 |
7.9–20.1 |
|
42.0 |
3.2 |
35.6–48.3 |
|
|
10 |
11.0 |
1.6 |
7.8–14.2 |
|
35.0 |
14.0 |
7.6–62.3 |
|
|
Liver metastasis |
|
No |
17.0 |
2.2 |
12.5–21.4 |
0.470 |
47.0 |
4.7 |
36.7–53.6 |
0.007 |
|
Yes |
15.0 |
- |
- |
|
2.0 |
0.8 |
0.4–3.6 |
|
|
Bone metastasis |
|
|
|
|
|
|
|
|
|
Yes |
17.0 |
1.2 |
14.6 – 19.3 |
0.040 |
42.0 |
2.9 |
36.2–47.7 |
0.100 |
|
No |
39.0 |
11.6 |
16.1 – 61.8 |
|
Not Reached |
- |
- |
|
|
No. of bone metastasis |
|
< 4 |
35.0 |
4.6 |
25.8–44.1 |
0.008 |
64.0 |
- |
- |
0.020 |
|
4–10 |
38.0 |
26.2 |
0.0–89.5 |
|
64.0 |
- |
- |
|
|
> 10 |
15.0 |
1.5 |
12.0–18.0 |
|
38.0 |
7.1 |
24.0–52.0 |
|
|
First-line therapy |
|
ADT |
19.0 |
4.5 |
10.1–27.8 |
0.014 |
- |
- |
- |
- |
|
CAB |
17.0 |
1.4 |
14.2–19.7 |
|
- |
- |
- |
|
|
ADT + docetaxel |
14.0 |
8.1 |
0.0–30.0 |
|
- |
- |
- |
|
|
Time to nadir PSA, months |
|
< 6 |
13.0 |
2.8 |
7.3–18.6 |
0.030 |
38.0 |
4.7 |
28.7–47.2 |
0.020 |
|
> 6 |
25.0 |
13.4 |
0.0–51.2 |
|
64.0 |
12.0 |
40.4–87.6 |
|
|
Minimum testosterone level |
|
< 0.7 nmol/L |
18.0 |
2.0 |
14.0–23.0 |
0.008 |
43.0 |
4.5 |
34.0–52.0 |
< 0.001 |
|
0.7–1.7 nmol/L |
10.0 |
- |
- |
|
12.0 |
- |
- |
|
|
mCRPC |
|
Yes |
- |
- |
- |
- |
42.0 |
2.2 |
37.6–46.3 |
0.003 |
|
No |
- |
- |
- |
- |
Not reached |
- |
- |
|
|
PSA at time of mCRPC |
|
< 10 |
- |
- |
- |
- |
53.0 |
11.0 |
31.4–74.5 |
0.030 |
|
11–20 |
- |
- |
- |
- |
64.0 |
- |
- |
|
|
> 20 |
- |
- |
- |
- |
34.0 |
8.4 |
17.4–50.5 |
|
|
Performance status at the time of mCRPC |
|
0 |
- |
- |
- |
- |
Not reached |
- |
- |
0.005 |
|
1 |
- |
- |
- |
- |
43.0 |
6.5 |
30.1–55.8 |
|
|
2 |
- |
- |
- |
- |
42.0 |
0.0 |
- |
|
|
3 |
- |
- |
- |
- |
18.0 |
2.6 |
12.8–23.1 |
|
*Continuous variables (e.g., age, PSA levels, etc) not included. Only categorical variables were included in this table.
CI: confidence interval; SE: standard error; TRUS: transrectal ultrasound-guided biopsy; TURP: transurethral resection of prostate; PSA: prostate-specific antigen; ADT: androgen deprivation therapy; CAB: combined androgen blockade; mCRPC: metastatic castration-resistant prostate cancer.
The OS post mCRPC was 17 months [Figure 3a]. On univariate analysis, age, visceral metastasis, PS at the time of mCRPC, treatment for mCRPC, treatment dose reductions [Figure 3b], treatment duration of first-line treatment for mCRPC, second-line treatment for mCRPC, and use of bone modifying agents significantly affected OS-2, while none of the factors were significant on multivariate analysis.
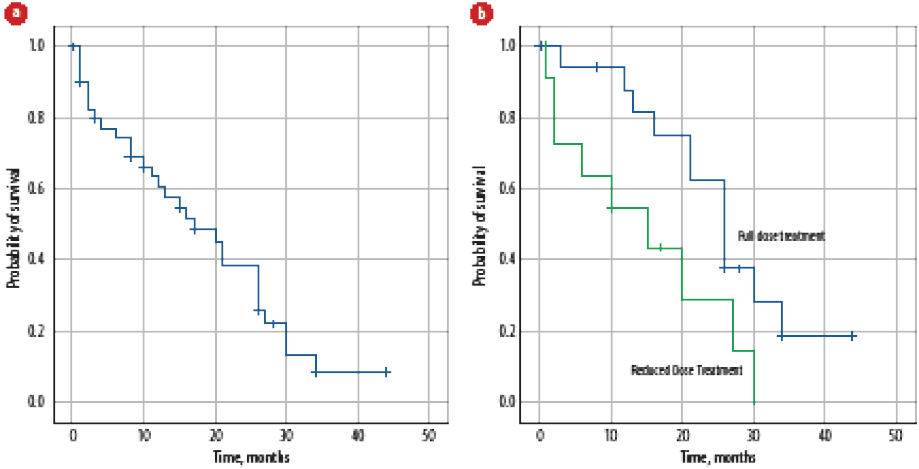
Figure 3: (a) Overall survival of patients with metastatic castration-resistant prostate cancer. (b) Effect of treatment dose reduction on overall survival of patients with metastatic castration-resistant prostate cancer.
Discussion
The Oman National Cancer Registry captures the incidence of all cancers every year, but it does not collect data about the clinical stage, patterns of metastases, prognostic factors, or outcome of cancer.5 For the first time, we report the outcome of Omani men with mPCa. The majority of patients presented with poor risk features (high Gleason score and serum PSA level > 20). Bone was the most common site of metastatic disease. The median PFS was 17.0 months, while the median OS was 43 months. The median survival post mCRPC was 17 months.
Increasing age is considered one of the risk factors for PCa and age-specific incidence increases with each decade of life.8 Reported median age at the time of diagnosis ranges between 62 and 73 years across different continents. Median age of Asian men diagnosed with PCa is higher than European Americans or African Americans.12 The median age at diagnosis in our cohort was 71 years. Our results are consistent with those reported from Europe, Asia, and Sub-Saharan Africa.3,11,13,14
Asian men with PCa have higher serum PSA levels, a higher Gleason score, and a higher clinical stage at diagnosis than European or American patients. Serum PSA in Asian men is also higher than African men living in America or Africa.12 Studies from India, Egypt, Hong Kong, Japan, and Nigeria also support these findings.3,11,14–16 Our results are consistent with these studies, as the median PSA level was 100.0 ng/mL. Similarly, 61.3% patients had Gleason scores of ≥ 8. The combination of PSA levels and Gleason scores were significantly higher compared to the studies reported from India, Nigeria, and Egypt.3,14,15
Median time to nadir PSA and nadir PSA levels have significant impact on the survival outcomes of PCa.11,16 Median nadir PSA level was 0.55 ng/mL with a median time of six months to nadir PSA in our cohort. The results are consistent with studies reported from Japan and Hong Kong, which revealed a median time to nadir PSA of 6–7.3 months.11,16 All these studies found nadir PSA levels and median time to nadir PSA significantly affecting the PFS and OS.11,16 A study from Nigeria,14 reported a nadir PSA of 4 ng/mL with a PFS and OS of 26.8 and 40.3 months, respectively. Although the median nadir PSA level was much higher in this study than our results (4.0 vs. 0.55 ng/mL), median PFS was longer in Nigerian men than our patients (26.8 vs. 17.0 months). However, OS was almost identical (40.3 vs. 43 months). These studies indicate the difference in PCa outcomes in different patient populations with various median nadir PSA levels.
The overwhelming majority of patients had bone metastases at the time of disease diagnosis, with nearly two-thirds of patients having > 10 bony lesions. Almost similar figures were reported from Egypt, India, and Hong Kong,3,15,16 though the number of patients and extent of bony lesions were lesser than our cohort.
Interest to treat the primary PCa even in the presence of metastatic disease increased after encouraging results from other diseases (e.g., breast, colon, and ovarian cancer).17 At least two randomized trials (HORRAD and STAMPEDE) and a meta-analysis showed the beneficial effect of radiotherapy to the primary site and improved OS by 7% at three years for patients with low metastatic disease burden (defined as < 5 bone metastases and no visceral disease).10 The STAMPEDE trial also showed improved PFS, failure-free survival, and prostate-specific survival in favor of localized radiotherapy, when added to the standard ADT. The National Comprehensive Cancer Network and European Society of Medical Oncology have incorporated the option of using radiotherapy with ADT for treating the primary site in patients with mHSPC with a low disease burden.10,18 Besides radiotherapy to the primary tumor, radical surgery to the primary site also improves five-year OS and disease-specific survival.17 In our cohort, only one patient was treated with ADT and radiotherapy, while two had radical surgery at the time of diagnosis because of low metastatic burden. Both HORRAD and STAMPEDE were reported in 2019 and 2018, respectively, while the cohort of patients described here includes patients from 2006 onwards, making it easier for a reader to understand the low number of cases.
Medical castration with either ADT, CAB, or surgical castration was the standard of care before the approval of docetaxel and newer hormonal agents for mPCa.19 In the last decade, treatment for PCa has taken significant strides. PFS and OS have increased significantly for patients with mHSPC with the addition of docetaxel and abiraterone earlier in the disease course.9,10 Risk of death reduced by 24% (STAMPEDE trial) to 27% (CHAARTED trial) with docetaxel and from 37% (STAMPEDE trial) to 38% (LATITUDE trial) with abiraterone.9,10 The vast majority of patients in our cohort were treated with either single-agent ADT or CAB. Only six (9.7%) patients received upfront docetaxel along with ADT. Upfront docetaxel or abiraterone were approved for mHSPC or high-risk PCa after 2018 while we report our cohort since 2006, hence it is easy to understand few patients being treated with upfront docetaxel in our report. We cannot compare our results to results reported from Egypt as all patients were treated with upfront docetaxel for mHSPC in that study.3 Due to small numbers treated with upfront docetaxel and shorter follow-up, it was too early to see the effect on PFS or OS.
Median PFS of mHSPC or time to the development of mCRPC is reported to be 18–24 months.9,20 Median time to CRPC was 17 months in our cohort, which is better than reported from India15 and Hong Kong,16 while considerably lower than that reported from Nigeria.14 Age, PSA level, and the number of bony metastasis were significant factors affecting the time to mCRPC in our study and a study reported from India,1 while median nadir PSA levels and median time to nadir PSA were significant factors as reported from Hong Kong and Nigeria.14,16 Treatment with docetaxel, abiraterone, and enzalutamide has improved the survival of patients with mCRPC.10 All three agents were used for our cohort once patients developed mCRPC (28 of 40 patients). It should be noted that whereas the median OS for mCRPC used to be 10–12 months before docetaxel and the hormonal agents,20 there has been a significant improvement with the introduction of these agents, and our results of median OS (OS-2) of 17 months is consistent with the published literature.9
The median OS for the studied cohort was 43 months, which is shorter than reported from India15 but longer than reported from Hong Kong.16 Various factors (patient PS, Gleason score, visceral metastases, PSA, hemoglobin, lactate dehydrogenase (LDH), albumin, and alkaline phosphatase at the time of diagnosis) were tested for predicting the outcome of patients with mCRPC treated with various agents enrolled on six different clinical trials in various settings.21 The prognostic score could predict four groups with the worst median survival of 8.8 months and longest survival of 22.8 months.21 Likewise, another group suggested a simpler prognostic score comprising PSA, LDH, neutrophil to lymphocyte ratio, and patient PS for a relatively small sample size of patients treated with abiraterone.22 On univariate analysis, Gleason Score, PSA levels, and PS significantly affected the OS in our cohort.
There are several limitations of our study. The sample size was small, and the cohort was treated over a long period, during which treatment options for mPCa have changed considerably. Although we report the outcome of Omani men with mPCa from a single centre, this still can be a representative sample for the country to a large extent as only two hospitals in the Sultanate provide cancer care and receive referrals from all over the country.
Conclusion
Omani men with mPCa present with high PSA levels, a higher cumulative Gleason score, and a high risk disease. The OS was comparable to reported outcomes from various regions of the world.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
references
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018 Nov;68(6):394-424.
- 2. Al-Abdin OZ, Al-Beeshi IZ. Prostate cancer in the Arab population: an overview. Saudi Med J 2018;39(5):453-458.
- 3. Alhanafy AM, Zanaty F, Ibrahem R, Omar S. Prognostic factors for hormone sensitive metastatic prostate cancer: impact of disease volume. Asian Pac J Cancer Prev 2018;19(4):1113-1118.
- 4. Taitt HE. Global trends and prostate cancer: a review of incidence, detection, and mortality as influenced by race, ethnicity, and geographic location. Am J Mens Health 2018;12(6):1807-1823.
- 5. Al-Bahrani BA, Al-Lawati NA, Al-Siyabi NH, Al-Gharbi DO, Al-Wehaibi. Cancer incidence in Oman 2015. Ministry of Health: Oman. 2015 [cited 2020 March]. Available from: https://www.moh.gov.om/documents/272928/1232802/Cancer+Incidence+in+Oman+2015/fdefdd19-47c3-e101-d953-9dafba9c650c.
- 6. Kumar S, Burney IA, Satyapal N, Kunju J, Al-Marhoon MS, Siddiqui KM. Clinicopathological features, treatment and outcome of Omani patients with localised prostate cancer. Arab J Urol 2020;18(4):219-225.
- 7. Al-Lawati JA, Al-Zakwani I, Fadhil I, Al-Bahrani BJ. Cancer incidence in Oman (1996-2015). Oman Med J 2019;34(4):271-273.
- 8. Kimura T, Egawa S. Epidemiology of prostate cancer in Asian countries. Int J Urol 2018 Jun;25(6):524-531.
- 9. Gravis G. Systemic treatment for metastatic prostate cancer. Asian J Urol 2019;6(2):162-168.
- 10. National Comprehensive Cancer Network. Prostate cancer version 1. 2020: national comprehensive cancer network; 2020 [cited 2020 April 26]. Available from: https://pubmed.ncbi.nlm.nih.gov/33545689/.
- 11. Kitagawa Y, Ueno S, Izumi K, Kadono Y, Mizokami A, Hinotsu S, et al. Clinical outcomes and nadir prostate-specific antigen (PSA) according to initial PSA levels in primary androgen deprivation therapy for metastatic prostate cancer. World J Urol 2016 Mar;34(3):319-327.
- 12. Zeigler-Johnson CM, Rennert H, Mittal RD, Jalloh M, Sachdeva R, Malkowicz SB, et al. Evaluation of prostate cancer characteristics in four populations worldwide. Can J Urol 2008;15(3):4056-4064.
- 13. Thomsen FB, Mikkelsen MK, Hansen RB, Krug AH, Glenthøj A, Stattin P, et al. Clinical characteristics and primary management of patients diagnosed with prostate cancer between 2007 and 2013: status from a Danish primary referral center. Acta Oncol 2016 Dec;55(12):1456-1460.
- 14. Bello JO. Predictors of survival outcomes in native sub Saharan black men newly diagnosed with metastatic prostate cancer. BMC Urol 2017;17(1):39.
- 15. Sureka SK, Maheshwari R, Agnihotri S, Mitash N, Ahmad S, Mandhani A. Predictors for progression of metastatic prostate cancer to castration-resistant prostate cancer in Indians. Indian J Med Res 2016;143(Supplement):S68-S73.
- 16. Teoh JY, Tsu JH, Yuen SK, Chan SY, Chiu PK, Lee WM, et al. Prognostic significance of time to prostate-specific antigen (PSA) nadir and its relationship to survival beyond time to PSA nadir for prostate cancer patients with bone metastases after primary androgen deprivation therapy. Ann Surg Oncol 2015 Apr;22(4):1385-1391.
- 17. Sinha S, Muralidhar V, Feng FY, Nguyen PL. Characteristics and national trends of patients receiving treatment of the primary tumor for metastatic prostate cancer. Prostate Int 2017;5(3):89-94.
- 18. ESMO Guidelines Committee. EUPDATE – Cancer of the prostate treatment recommendations. [cited 2020 April 29]. Available from: https://doi.org/10.1016/j.annonc.2020.06.011.
- 19. Parker C, Gillessen S, Heidenreich A, Horwich A; ESMO Guidelines Committee. Cancer of the prostate: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015 Sep;26(Suppl 5):v69-v77.
- 20. Petrylak DP, Tangen CM, Hussain MH, Lara PN Jr, Jones JA, Taplin ME, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med 2004 Oct;351(15):1513-1520.
- 21. Halabi S, Small EJ, Kantoff PW, Kattan MW, Kaplan EB, Dawson NA, et al. Prognostic model for predicting survival in men with hormone-refractory metastatic prostate cancer. J Clin Oncol 2003 Apr;21(7):1232-1237.
- 22. Boegemann M, Schlack K, Früchtenicht L, Steinestel J, Schrader AJ, Wennmann Y, et al. A prognostic score for overall survival in patients treated with abiraterone in the pre- and post-chemotherapy setting. Oncotarget 2019;10(49):5082-5091.