Anaphylaxis is a serious allergic reaction that develops suddenly after exposure to an allergen or trigger and can be life-threatening.1,2 While it is usually multisystemic, severe involvement of a single system may occur. Various criteria have been developed to help identify and diagnose anaphylaxis.3,4 The reaction is usually caused by type I immune-mediated hypersensitivity via the production of specific immunoglobulin (Ig) E and mediators released from mast cells and basophils.5,6 Specific IgE is produced due to a Th2-skewed immune response and is important for crosslinking IgE receptors present on the surface of mast cells, the effector cells for type I allergies or immediate hypersensitivity-mediated reactions such as anaphylaxis. Subsequently, the mast cells release mediators responsible for the clinical features of anaphylaxis, primarily histamine, tryptase, leukotrienes, and prostaglandins.7–9
The most common causes and triggers of anaphylaxis in children are cow’s milk, egg, fish, soy, wheat, peanuts, tree nuts, and seafood. In contrast, the most frequent food triggers in adults are peanuts, tree nuts, and seafood.10 Other non-food-related anaphylactic triggers include insect venom (i.e., following bites or stings from insects like honeybees, hornets, yellow jacket wasps, and ants), medications (such as penicillin, cephalosporin, aspirin, and anesthetics), biological agents, exercise, and latex which can cause anaphylaxis during surgical procedures.11–13 However, in some cases, the exact cause or trigger of the allergic reaction is unknown; this is referred to as idiopathic anaphylaxis.14 Patients with anaphylaxis usually present initially to the emergency department, after which they may require further allergy evaluation. Alternatively, some patients present directly to allergy or immunology clinics for evaluation and diagnosis following one or more anaphylactic episodes.
Anaphylaxis usually manifests clinically with cutaneous symptoms such as redness or flushing, urticarial rashes, and angioedema of the lips, face, or limbs. Moreover, the angioedema may involve the tongue, throat, and larynx, thus putting the patient at risk of upper airway obstruction.5 Other symptoms of anaphylaxis can affect the lungs, such as wheezing, severe bronchospasms, and the inability to breathe, leading to respiratory arrest. Gastrointestinal symptoms such as cramps, abdominal pain, and vomiting may also be a sign of anaphylaxis, usually due to bowel edema, while cardiovascular symptoms can include tachycardia, hypotensive shock, and fatal arrhythmias.5 Certain patients are at increased risk of fatal anaphylaxis because of an underlying chronic disease or risk factors (such as untreated asthma) or the use of beta-blockers for heart disease.1,3
The initial treatment of anaphylaxis consists of ensuring the airway, breathing, and circulation, followed by administering injectable epinephrine.15 Up to 20% of patients develop biphasic anaphylaxis and experience a second anaphylactic episode approximately 4–6 hours after the initial episode due to a secondary allergic response. As such, most patients are usually monitored in the emergency department for 6–8 hours after treatment before being discharged.16 Primary prevention is the best management option for patients at risk of developing anaphylaxis; however, pre-hospital management is recommended as accidental exposure to allergenic agents does occur and pose a risk.17 Once diagnosed by an allergy specialist, patients are prescribed self-injectable epinephrine.18,19 Patients with known allergies should be instructed on using these autoinjector devices and carry them on their person at all times.20 In addition to the risk of mortality, anaphylaxis constitutes a burden to the healthcare system as these patients require emergency management, observations, intensive care, and outpatient care.21
While the spectrum of anaphylaxis is well-reported in Western countries, very few studies have evaluated the manifestations, triggers, and treatment of this condition in the Middle Eastern region.22–24 Moreover, to the best of our knowledge, no studies on this topic have yet been reported from Oman. As such, this study aimed to describe the clinical features, causes, investigation, and management of anaphylaxis among patients presenting to a tertiary care center in Oman.
Methods
This retrospective observational study included all patients diagnosed with anaphylaxis and seen at the allergy and immunology clinic of the Sultan Qaboos University Hospital (SQUH), Muscat, Oman, between August 2005 and June 2020. The allergy/immunology clinic at SQUH is a subspecialty tertiary care clinic, patients were referred from different institutions all over the country or the SQUH Emergency Department for evaluation due to a recent history of allergic reaction, including anaphylaxis. During their visit to the clinic, the patients underwent routine history-taking, physical examination, and allergy testing such as the skin prick test or measurement of specific IgE levels to confirm the allergy, if necessary. In all cases, a diagnosis of anaphylaxis was made based on clinical symptoms and the degree of systemic involvement. Patients with allergic symptoms and signs involving ≥ 2 or severe one system involvement are diagnosed with anaphylaxis as per previously published criteria.3 Patients who did not meet the diagnostic criteria for anaphylaxis were not included in the study.
Data were retrieved from the patients’ medical records and prescription history using the hospital information system. Sociodemographic and clinical information were collected, including age, sex, personal history of allergies, presence of other allergic conditions (i.e., asthma, rhinitis, or eczema), family history of allergies, total IgE level, eosinophil count, and whether the patient had been prescribed self-injectable epinephrine. In addition, the body system affected was noted based on clinical symptoms and categorized into cutaneous (urticaria, angioedema, or flushing), respiratory (wheezing, coughing, difficulty in breathing, throat itchiness, or stridor), cardiovascular (palpitations or hypotension/syncope), gastrointestinal (vomiting, abdominal pain, or diarrhea), and central nervous system (dizziness, unconsciousness or collapse) manifestations. Finally, the cause of the anaphylaxis was classified as either food (cow’s milk, eggs, fish, peanuts, tree nuts, and seafood), insect venom (honeybees, wasps, or ants), medications, latex, exercise, contrast media, or blood products. Cases in which the exact cause of anaphylaxis was unknown were considered to indicate idiopathic anaphylaxis.
The data were entered into an electronic datasheet before being exported to SPSS (IBM Corp. Released 2015. IBM SPSS Statistics for Windows, Version 23.0. Armonk, NY: IBM Corp.) for statistical analysis. The results were presented using simple descriptive statistics such as frequencies and percentages. The means and standard deviation between groups and continuous variables were compared using one-way analysis of variance (ANOVA), and the difference between categorical variables was compared using Pearson’s chi-square test. The differences were considered statistically significant if the p-value was < 0.050. The study received ethical approval from the Medical Research and Ethics Committee of the College of Medicine and Health Sciences, Sultan Qaboos University, Muscat, Oman #1549 dated 8 October 2017.
Results
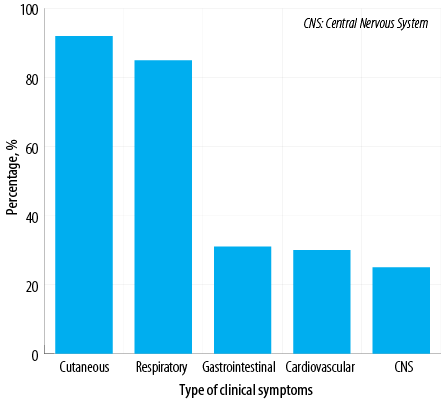
Figure 1: Systemic involvement according to clinical symptoms among Omani patients diagnosed with anaphylaxis (N = 100).
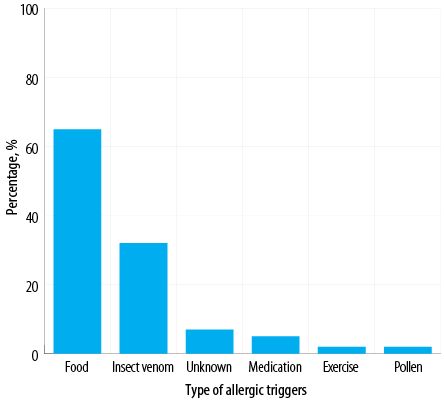
Figure 2: Anaphylactic causes or triggers identified among Omani patients diagnosed with anaphylaxis (N = 100).
A total of 100 patients were diagnosed with anaphylaxis during the study period. Of these, 52.0% were male, and 48.0% were female. The patients ranged in age from three months to 57 years old, with a mean and median of 15.9±16.2 and 10.0 (2.9–26.5) years, respectively. Overall, 70.0% of patients were < 18 years old. A personal history of allergies was reported by 66.0% of patients, with many patients additionally reporting allergic diseases such as asthma (43.0%), rhinitis (29.0%), or eczema (42.0%). A family history of allergies was positive in 72.0% of patients. The eosinophil count ranged from 0.0–16.9 × 109/L, with a mean of 0.8±2.2 × 109/L and a median of 0.3 (0.15–0.6) × 109/L. Total IgE levels ranged from 25–8,706 kIU/L, with a mean of 935.1±1369.5 kIU/L and a median of 500.4 (186.0–972.5) kIU/L. Self-injectable epinephrine was prescribed to all patients [Table 1].
Table 1: Characteristics of Omani patients diagnosed with anaphylaxis (N = 100).
|
Age at diagnosis, years |
|
|
Median (range) |
10.0 (0.2–57.4) |
|
Mean |
15.9 |
|
Sex |
|
|
Male |
52.0 |
|
Female |
48.0 |
|
Family history of allergy |
|
|
Yes |
72.0 |
|
No |
28.0 |
|
Personal history of allergy |
|
|
Yes |
66.0 |
|
No |
44.0 |
|
Presence of other allergic conditions |
|
Asthma |
43.0 |
|
Rhinitis |
29.0 |
|
Eczema |
42.0 |
|
Eosinophil count, × 109/L |
|
|
Median (range) |
0.3 (0.0–16.9) |
|
Mean |
0.8 |
|
Total IgE, kIU/L |
|
|
Median (range) |
500.4 (25.0–8706.0) |
|
Mean |
935.1 |
|
Prescribed injectable epinephrine |
The most common bodily system involved was the skin (92.0%), followed by the respiratory system (85.0%), gastrointestinal system (31.0%), cardiovascular system (30.0%), and central nervous system (25.0%) [Figure 1]. The majority of patients (87.0%) demonstrated the involvement of more than one system. The most frequent cause of anaphylaxis was food (65.0%), followed by insect venom (32.0%), medications (5.0%), and pollen (2.0%). In addition, there were two cases of exercise-induced anaphylaxis and seven cases of idiopathic anaphylaxis [Figure 2]. Among patients with food-related anaphylaxis, specific triggers included tree nuts (38.0%), eggs (25.0%), peanuts (19.0%), shrimp (17.0%), cow’s milk (14.0%), and fish (10.0%). For those with anaphylaxis triggered by venom, the insects responsible were primarily black ants (21.0%), followed by wasps (9.0%) and honeybees (6.0%).
In the majority of patients (81.0%), anaphylaxis was triggered by a single cause. However, in 12.0% of cases, two to three causes of anaphylaxis were identified, whereas the number of causes could not be determined in the remaining 7.0% of cases. Overall, total IgE levels did not differ considerably between patients according to the number of causes of anaphylaxis [Figure 3a]; single cause mean was 1072.9 (188.0) kIU/L, median of 510.7 (212.8–1259.3) kIU/L, two or more causes mean was 521.6 (106.3) kIU/L, median of 597.6 (82.4–810.6) kIU/L, and unknown mean was 223.9 (81.5) kIU/L, median of 171.0 (66.5–379.1) kIU/L (all p-values > 0.050). The highest values were seen in the category of single cause anaphylaxis. Total IgE levels were significantly elevated for the group of patients who also had another allergic diseases, such as asthma, rhinitis, or eczema (mean of 1193.8 (227.1) kIU/L, with median of 594.4 (239.8–1359.2) kIU/L) compared to patients without another concomitant allergic disease (mean of 503.6 (110.0) kIU/L, with median of 288.6 (109.8–615.3) kIU/L, p = 0.030)) [Figure 3b]. In addition, concomitant other allergic disease is significantly higher in patients < 18 years old (75.4%) compared to patients >18 years of age (45.2%, p = 0.010).
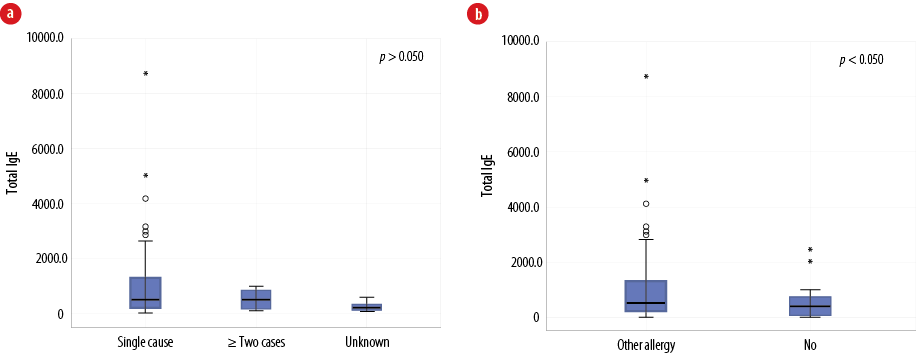
Figure 3: (a) The relationship between total immunoglobulin (Ig) E level and the number of identified allergic triggers among Omani patients diagnosed with anaphylaxis (N = 100). (b) The relationship between total IgE level and the presence of other allergic conditions such as eczema, asthma, and rhinitis in patients with anaphylaxis (N = 100).
Discussion
This is the first study from Oman to evaluate and describe the clinical spectrum and management of anaphylaxis. Most of the patients were children or adolescents < 18 years of age, and with almost similar number of male and female patients. Moreover, it would seem that the patients were highly atopic, given that the majority had a positive family history of allergies in addition to a personal history of other allergic conditions, such as asthma, rhinitis, and eczema. These findings are expected given the natural history or progression of allergic diseases (i.e., atopic march).25 Moreover, while both the mean and median eosinophil count were below the upper limit of normal, total IgE levels were elevated more in patients who also have another concomitant allergic disease. The presence of other allergic diseases is more significant in children compared to adults. This is consistent with the notion that atopy predisposes patients to allergy and anaphylaxis and the
atopic march.26
The commonest clinical manifestations of anaphylaxis were cutaneous symptoms (urticaria or angioedema) followed by respiratory and gastrointestinal symptoms. This is in line with findings published for other populations in the Middle East and elsewhere around the world.22,27,28 Nevertheless, a small proportion of our patients (8.0%) did not manifest cutaneous symptoms. This finding is important as a severe allergic reaction or anaphylaxis may not be suspected without cutaneous symptoms, thus delaying treatment and potentially resulting in the patient going into hypotensive shock. Indeed, up to 10% of anaphylactic patients do not show cutaneous symptoms or may manifest such symptoms only after a delayed onset.29 On the other hand, cutaneous, mucosal, or gastrointestinal symptoms alone are sometimes not considered sufficient for a diagnosis of anaphylaxis in the absence of other signs like respiratory compromise or cardiovascular symptoms.4,29 A diagnosis of anaphylaxis is mainly based on clinical symptoms and associations with a known trigger; however, specific allergy testing may be required in doubtful cases, such as skin prick testing, evaluation of specific IgE levels or serum tryptase, and challenge procedures in controlled settings.4
Food triggers represented the most frequent cause of anaphylaxis, with specific allergens being tree nuts, eggs, peanuts, shrimp, cow’s milk, and fish. Similar causes of food-induced anaphylaxis have been reported elsewhere.28 The prevalence of food allergies and specific food triggers of anaphylaxis may vary from one country or region to another, depending on cultural variations in popular foods.5 In general, food allergies are more common in children than adults, as many individuals outgrow their allergies as they get older.30 Insect venom was the second most common causative factor of anaphylaxis in the present study; black ants were the most frequent culprit. Black ants have been reported to cause significant allergy-related morbidity in other studies from the Middle East, causing both systemic and local reactions.3,4,31 Insect bites or stings require a special attention as the resulting allergies are known to be severe or even fatal, particularly as emergency services may not arrive in time before the patient goes into hypotensive shock.3 Appropriate diagnosis and rapid intervention are essential to reduce morbidity and mortality secondary to insect-related systemic allergies.
Idiopathic anaphylaxis was observed in 7.0% of the present cohort. Other studies have revealed that this type of anaphylaxis affects up to 20% of patients.3,4 Idiopathic anaphylaxis represents a challenge for both the affected patient and the allergist as the causative trigger of the allergic response cannot be identified. Unfortunately, a diagnosis of idiopathic anaphylaxis precludes avoidance of the trigger; therefore, such patients need to rely solely on self-injectable epinephrine. Other allergic triggers were observed in fewer patients in the current study, including medications, exercise, and pollen. Medication allergies can constitute a significant diagnostic obstacle for the allergist as standardized diagnostic testing materials are seldom available, except penicillin.32 Moreover, in the absence of a safer alternative, desensitization is the only acceptable intervention if the reaction is mediated through an IgE mechanism.7,8
We identified more than one cause of anaphylaxis in some patients, such as both food and insect venom. This is probably related to the tendency of certain patients to develop allergic diseases. Indeed, while total IgE levels were similar between patients with different causes of anaphylaxis, they were more elevated in the group of patients who also had other allergic diseases, such as asthma, rhinitis, and eczema and the highest values were seen in later group and patients with a single cause of anaphylaxis. It is well established that total IgE is widely variable among patients with allergic diseases, particularly in patients with polysensitization.33 Overall, the ideal management of allergic disease and anaphylaxis is strict avoidance of the trigger, if known. Hence, an attempt should always be made to identify the culprit, particularly in difficult cases or patients with a history of allergic episodes. However, even when triggers are known, accidental exposure to the allergen can occur for various reasons, such as accidental cross-contamination in food allergies or unavoidable contact with flying insects. As such, international guidelines recommend self-injectable epinephrine as a first-line treatment or secondary prevention measure for all patients with a history of anaphylaxis.20 In the current study, all the patients were prescribed and instructed to keep autoinjector devices with them at all times after receiving a demonstration on how to use the device in an emergency.
Delays in the administration of epinephrine are associated with increased morbidity and mortality in anaphylactic patients.34,35 Moreover, using self-injectable epinephrine as a form of pre-hospital management gives affected patients sufficient time to reach an emergency department where further management and repeated doses can be administered, alongside adjunctive therapy such as antihistamines and glucocorticosteroids.17,34 Epinephrine decreases airway edema and smooth muscle spasms; in addition, it has inotropic and chronotropic effects, which counter the effects of hypotension and shock, and reduce hospitalization and fatality rates.34 In cases of anaphylaxis, there is no absolute contraindication to use epinephrine due to the risk to the patient’s life; however, the pharmacological effect of the drug is similar to those seen with the fight or flight physiological response, including tremors, anxiety, transient pallor, and palpitations.17–19
This study was conducted in a controlled setting where patients are highly atopic with allergy manifestation and received specialized diagnostic services. Furthermore, the patients were referred from different governorates in the country, possibly reflecting the situation at large. The limitation of this study was its retrospective nature and single-center; furthermore, possibly not all patients presenting at primary and secondary healthcare institutions were referred.
Further research is recommended to determine the true incidence and prevalence of anaphylaxis in Oman to better understand the burden of the condition and help establish initiatives to minimize associated morbidity and mortality. To this end, a multicenter study of patients presenting to emergency services would allow a better picture of the impact of anaphylaxis on the national healthcare system. In contrast, a population-based study would more accurately reflect the incidence and prevalence of this condition within the community as some patients may not present to a healthcare facility.
Conclusion
Anaphylaxis represents a life-threatening emergency for the affected patient; therefore, awareness of the epidemiological patterns and clinical features of this condition in specific populations is important to establish appropriate management strategies and ensure rapid treatment. This study is the first in Oman to describe anaphylaxis in a group of patients seen at a tertiary care hospital. While the clinical features and results of the cohort were similar to those reported elsewhere in the literature, these findings establish an important baseline for future studies.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
Acknowledgements
We would like to thank all doctors who referred patients to our clinic and thank all patients for their trust.
references
- 1. Yunginger JW, Sweeney KG, Sturner WQ, Giannandrea LA, Teigland JD, Bray M, et al. Fatal food-induced anaphylaxis. JAMA 1988 Sep;260(10):1450-1452.
- 2. Pouessel G, Claverie C, Labreuche J, Dorkenoo A, Renaudin JM, Eb M, et al. Fatal anaphylaxis in France: analysis of national anaphylaxis data, 1979-2011. J Allergy Clin Immunol 2017 Aug;140(2):610-612.e2.
- 3. Sampson HA, Muñoz-Furlong A, Bock SA, Schmitt C, Bass R, Chowdhury BA, et al. Symposium on the definition and management of anaphylaxis: summary report. J Allergy Clin Immunol 2005 Mar;115(3):584-591.
- 4. Muraro A, Roberts G, Worm M, Bilò MB, Brockow K, Fernández Rivas M, et al; EAACI Food Allergy and Anaphylaxis Guidelines Group. Anaphylaxis: guidelines from the European academy of allergy and clinical immunology. Allergy 2014 Aug;69(8):1026-1045.
- 5. Simons FE. Anaphylaxis. J Allergy Clin Immunol 2010 Feb;125(2)(Suppl 2):S161-S181.
- 6. Shida K, Hachimura S, Ametani A, Ishimori M, Ling M, Hashiguchi M, et al. Serum IgE response to orally ingested antigen: a novel IgE response model with allergen-specific T-cell receptor transgenic mice. J Allergy Clin Immunol 2000 Apr;105(4):788-795.
- 7. Lieberman P, Garvey LH. Mast cells and anaphylaxis. Curr Allergy Asthma Rep 2016 Mar;16(3):20.
- 8. Yamaguchi M, Lantz CS, Oettgen HC, Katona IM, Fleming T, Miyajima I, et al. IgE enhances mouse mast cell Fc(epsilon)RI expression in vitro and in vivo: evidence for a novel amplification mechanism in IgE-dependent reactions. J Exp Med 1997 Feb;185(4):663-672.
- 9. Reber LL, Hernandez JD, Galli SJ. The pathophysiology of anaphylaxis. J Allergy Clin Immunol 2017 Aug;140(2):335-348.
- 10. Worm M, Grünhagen J, Dölle S. [Food-induced anaphylaxis - data from the anaphylaxis registry]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2016 Jul;59(7):836-840.
- 11. Fiedler C, Miehe U, Treudler R, Kiess W, Prenzel F. Long-term follow-up of children after venom immunotherapy: low adherence to anaphylaxis guidelines. Int Arch Allergy Immunol 2017;172(3):167-172.
- 12. Pravettoni V, Incorvaia C. Diagnosis of exercise-induced anaphylaxis: current insights. J Asthma Allergy 2016 Oct;9:191-198.
- 13. Manuyakorn W, Benjaponpitak S, Kamchaisatian W, Vilaiyuk S, Sasisakulporn C, Jotikasthira W. Pediatric anaphylaxis: triggers, clinical features, and treatment in a tertiary-care hospital. Asian Pac J Allergy Immunol 2015 Dec;33(4):281-288.
- 14. Greenberger PA, Lieberman P. Idiopathic anaphylaxis. J Allergy Clin Immunol Pract 2014 May-Jun;2(3):243-250.
- 15. Sicherer SH, Simons FE; SECTION ON ALLERGY AND IMMUNOLOGY. Epinephrine for first-aid management of anaphylaxis. Pediatrics 2017 Mar;139(3):e20164006.
- 16. Tole JW, Lieberman P. Biphasic anaphylaxis: review of incidence, clinical predictors, and observation recommendations. Immunol Allergy Clin North Am 2007 May;27(2):309-326.
- 17. Tiyyagura GK, Arnold L, Cone DC, Langhan M. Pediatric anaphylaxis management in the prehospital setting. Prehosp Emerg Care 2014 Jan-Mar;18(1):46-51.
- 18. Diwakar L, Cummins C, Ryan R, Marshall T, Roberts T. Prescription rates of adrenaline auto-injectors for children in UK general practice: a retrospective cohort study. British Journal of General Practice 2017;67(657):e300-e305.
- 19. Pouessel G, Deschildre A, Castelain C, Sardet A, Sagot-Bevenot S, de Sauve-Boeuf A, et al. Parental knowledge and use of epinephrine auto-injector for children with food allergy. Pediatr Allergy Immunol 2006 May;17(3):221-226.
- 20. Simons FE, Ebisawa M, Sanchez-Borges M, Thong BY, Worm M, Tanno LK, et al. 2015 update of the evidence base: world allergy organization anaphylaxis guidelines. World Allergy Organ J 2015 Oct;8(1):32.
- 21. Patel DA, Holdford DA, Edwards E, Carroll NV. Estimating the economic burden of food-induced allergic reactions and anaphylaxis in the United States. J Allergy Clin Immunol 2011;128(1):110-115.e5.
- 22. Sheikh F, Amin R, Rehan Khaliq AM, Al Otaibi T, Al Hashim S, Al Gazlan S. First study of pattern of anaphylaxis in a large tertiary care hospital in Saudi Arabia. Asia Pac Allergy 2015 Oct;5(4):216-221.
- 23. Mohammed Elhassan S, Charlson M, Jama H, Zakri F, Elajez RH, Ahmed F, et al. Management of anaphylaxis in children: a survey of parents and school personnel in Qatar. BMJ Paediatr Open 2017 Oct;1(1):e000077.
- 24. Alkanhal R, Alhoshan I, Aldakhil S, Alromaih N, Alharthy N, Salam M, et al. Prevalence triggers and clinical severity associated with anaphylaxis at a tertiary care facility in Saudi Arabia: a cross-sectional study. Medicine (Baltimore) 2018 Aug;97(31):e11582.
- 25. Hill DA, Spergel JM. The atopic march: critical evidence and clinical relevance. Ann Allergy Asthma Immunol 2018 Feb;120(2):131-137.
- 26. Lau S, Matricardi PM, Wahn U, Lee YA, Keil T. Allergy and atopy from infancy to adulthood: messages from the German birth cohort MAS. Ann Allergy Asthma Immunol 2019 Jan;122(1):25-32.
- 27. Poziomkowska-Gęsicka I, Kurek M. Clinical manifestations and causes of anaphylaxis. analysis of 382 cases from the anaphylaxis registry in west Pomerania province in Poland. Int J Environ Res Public Health 2020 Apr;17(8):2787.
- 28. Gonzalez-Estrada A, Silvers SK, Klein A, Zell K, Wang XF, Lang DM. Epidemiology of anaphylaxis at a tertiary care center: a report of 730 cases. Ann Allergy Asthma Immunol 2017 Jan;118(1):80-85.
- 29. Anagnostou K, Turner PJ. Myths, facts and controversies in the diagnosis and management of anaphylaxis. Arch Dis Child 2019 Jan;104(1):83-90.
- 30. Savage J, Johns CB. Food allergy: epidemiology and natural history. Immunol Allergy Clin North Am 2015 Feb;35(1):45-59.
- 31. Al-Shahwan M, Al-Khenaizan S, Al-Khalifa M. Black (samsum) ant induced anaphylaxis in Saudi Arabia. Saudi Med J 2006 Nov;27(11):1761-1763.
- 32. Kuruvilla M, Khan DA. Anaphylaxis to drugs. Immunol Allergy Clin North Am 2015 May;35(2):303-319.
- 33. Kuperstock JE, Brook CD, Ryan MW, Platt MP. Correlation between the number of allergen sensitizations and immunoglobulin E: monosensitization vs polysensitization. Int Forum Allergy Rhinol 2017 Apr;7(4):385-388.
- 34. Ellis AK, Day JH. The role of epinephrine in the treatment of anaphylaxis. Curr Allergy Asthma Rep 2003 Jan;3(1):11-14.
- 35. Simons FE. First-aid treatment of anaphylaxis to food: focus on epinephrine. J Allergy Clin Immunol 2004 May;113(5):837-844.