|
Abstract
Objectives: The main objective of the study was to examine the self reported health status in patients with ankylosing spondylitis (AS) compared with the general population and the secondary objective (in the AS group) was to study the association between health status, demographic parameters, and specific disease instruments in AS.
Methods: A cross sectional study of 100 AS patients recruited between 2006 and 2009 at the Department of Rheumatology. Health status was assessed by using the SF-36 health questionnaire in patients with AS. Demographic characteristics and disease specific instruments were also examined by the questionnaire. A sample of 112 healthy individuals was also surveyed using the SF-36 health questionnaire.
Results: This study showed a great impairment in the quality of life of patients with AS involving all scales. All male patients with AS reported significantly impaired health-related quality of life on all items of the SF-36 compared with the general population whereas female patients reported poorer health on three items only, namely physical functioning, general health and bodily pain. Mental health was mostly affected than physical role. The physical role was significantly higher in patients with high education level than in patients with low education level (p=0.01). Physical functioning was better in employed patients. All scales of SF-36 were correlated with BASFI, BASDAI and BAS-G. Only physical functioning and general health were correlated with BASMI.
Conclusion: Impairment in the quality of life can be significantwhen suffering from AS, affecting mental health more than physicalhealth. Among disease parameters, functional impairment,disease activity, mobility limitation, and spinal pain were the most associated factors resulting to the deterioration of quality of life.
Keywords: Ankylosing Spondylitis; Quality of Life; Generic Instrument; SF-36.
Introduction
Ankylosing spondylitis (AS) is a chronic inflammatory disorder primarily affecting the sacroiliac joints, axial skeleton and thoracic cage.1 Pain, joint stiffness, and a progressive loss of spinal mobility are the major symptoms of this disease leading to severe functional limitations.2 Quality of life and health status of patients with AS can be widely impaired by the disease. In fact, pain, functional limitations, spine, and articulation deformities cause issues not only on physical health but also on psychological wellbeing and social life.3 Recently, several methods of evaluation of disease activity, functional and metrological limitations were developed; however, they do not assess quality of living.1-4 The Short Form-36 (SF-36) is a generic instrument covering a wide range of quality of life dimensions. It can be used in different diseases and conditions and even in healthy controls.5,6 The main objective of this study was to examine the self-reported health status in patients with AS compared with the general population and secondary objectives (in AS group) were to explore the associations between health status, demographic parameters and specific disease instruments in AS.
Methods
This is a cross sectional study conducted on 100 consecutive patients with AS (89 men and 11 women) according to the modified New York criteria,7 recruited between 2006 and 2009 at the Department of Rheumatology. All patients were examined by a rheumatologist. Patients with spondylarthropathies other than AS were excluded as well as patients having associated diseases affecting quality of life such as diabetes, hearing or comprehension disorders.
Healthy indviduals (controls) were recruited from two dispensaries, Douar Hicher (urban region) and Sanhaja (rural region) of the district of Manouba. Data collected from 112 subjects were used for comparison. A random draw was performed from the National Register of Tunisia including the Tunisian population at the census of 2004. The sample was considered to reflect the general sex, age and education level distribution of the Tunisian population.
Health status was assessed by the SF-36 health survey in patients with AS and controls. Demographic characteristics and some disease-specific instruments were also examined by the questionnaire given to the patients. The study was approved by the institutional ethical committee for medical research.
Age, gender, educational level (<13 or >13 years of education), urban distribution (urban zone corresponds to more than 2000 inhabitants) and employment status (employed, unemployed, licensed) were collected. Disease duration, age at the onset of disease and clinical manifestations were assessed in the patient group.
The MOS SF-36 is a generic instrument providing data which may be presented as a profile describing quality of life in each of eight separate dimensions of self-reported health status.8-10 Each scale generates a score from 0 to 100, with a high score indicating a better quality of life and a low score indicating a worse quality of life.11 A validated Arabic version of the SF-36 was used,12 which has equivalent psychometric properties with the English version.13
Disease-specific instruments were used to evaluate respectively:
1. The disease activity by the Bath AS Disease Activity Index (BASDAI)14 consists of six visual analog scales (VAS) dealing with fatigue, spinal and peripheral joint pain, localized tenderness and morning stiffness (both qualitative and quantitative). Each VAS is scored from 0 to 10 (0=best, 10= worst score). The mean of the two scores relating to morning stiffness is calculated providing an aggregate score. The resulting score for the overall index (0-50) is converted to a 0-10 scale, providing the final BASDAI score.
2. The functional impairment by the Bath AS Disease Functional Index (BASFI)15 is a measure which includes ten items asking the respondents about the perception of their functional ability and how well they are able to function in everyday life. Subjects rate their functional ability from 0-10 on a VAS. The mean of the ten scales gives the BASFI score (range: 0-10).
3. The spinal and hip mobility by the Bath AS Metrology Index (BASMI)16 includes measures for cervical rotation, tragus to wall distance, lumbar side flexion, lumbar flexion (Schober’s index) and intermalleolar distance (score range: 0-10).
4. The impact of the disease on the general state of the patients by the Bath AS Disease Global Index (BASG-s)17 consists of two questions which asks patients to indicate on a VAS the effects that the disease has on their well being over the last week and the last six months (score range 0-100).
The data were analyzed using the Statistical Package for the Social Sciences (SPSS) 13.0 for Windows. Descriptive statistics are given as means and standard deviation. Differences between patients and controls were examined by chi-squared test for categorical variables. A sample t test was used to compare the mean scores of the SF-36 scales in the patient group and controls
For the possible relationships between the independent variables (age, gender, educational level, urban or rural habitation) and the results of quality of life assessed by the SF-36 questionnaire, the analysis of variance (ANOVA) was used as an adjustment test (scales of SF-36 were considered as a dependent variables). Within the patient group, two sample independent t tests were used for comparison of continuous variables. For comparison between educational groups, the sample was divided into a low educational group (≤13 years of education) and a high educational group (>13 years of education). A Pearson correlation analysis (r: Pearson coefficient) was performed between the disease specific instruments (BASDAI, BASFI, BAS-G, BASMI) and the items in the SF-36. The level of statistical significance (p) was set at 0.05.
Results
Eighty nine men and eleven women affected by AS were included in this study. The characteristics of the patients are summarized in Table 1. They were compared to 112 controls (50 men and 62 women). The mean age and region distribution of patients with AS were comparable to controls (p=0.3 and p=0.94, respectively). The proportion of men was higher in patients with AS than controls (p<0.001). High education level (>13 years) was more frequent in controls than patients (p<0.001).
Since AS patients and controls were different in some characteristics (Table 1), an ANOVA analysis was performed to explore the possible interactions between gender, educational level, urban distribution, employment condition and the presence or absence of AS. Male patients reported more impaired health than men in controls, across all scales of SF-36. Whereas, female patients reported significantly worse health across only three scales of SF-36 (physical functioning, general health, bodily pain), (Fig. 1). The controls from rural regions reported better health across all domains than patients with AS living in such regions. A similar report was found in urban region, (Fig. 2). Concerning the influence of level of education and quality of life in patients and controls we proceed to the calculation of standard difference SF-36 scores, it was a score based on the difference of scores between patients with AS and controls for each scales of the SF-36 and that separately for high and low education groups; then, the standard difference SF-36 scores of high and low education groups were compared. The high education group of patients reported significantly worse health across all scales of the SF-36 than controls except for mental health scale. Similar report was found in low education group. Domains of physical functioning, physical role, bodily pain and social functioning were significantly more impaired in patients with high education compared to low the education group. However, domains of role emotional, vitality and general health were significantly more impaired in the low education group compared to highly educated patients. (Fig. 3)
Table 1: Characteristics of Patients with AS and Control Subjects.
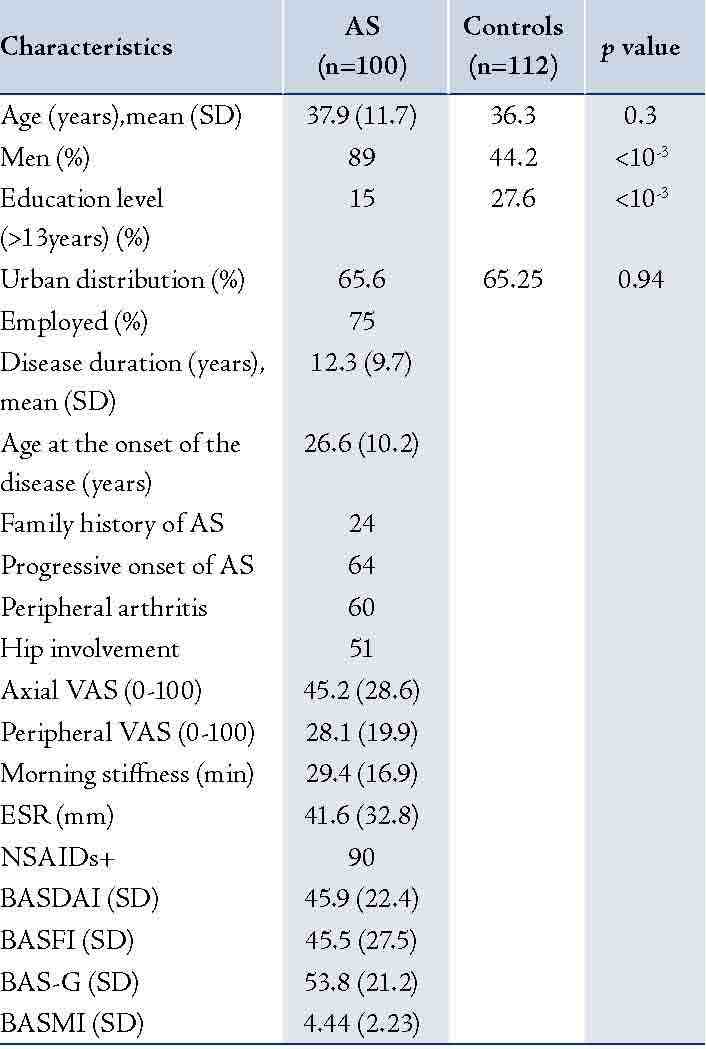
SD: Standard deviation; AS: Ankylosing Spondylitis; VAS: visual analogue scale; ESR: Erythrocyte Sedimentation Rate; NSAID: Nonsteroidal anti-inflammatory drugs; BASFI: Bath Ankylosing Functional Index; BASDAI: Bath Ankylosing Disease Activity Index; BAS-G: Bath Ankylosing Spondylitis Patient Global Score; BASMI: Bath Ankylosing Spondylitis Metrology Index.
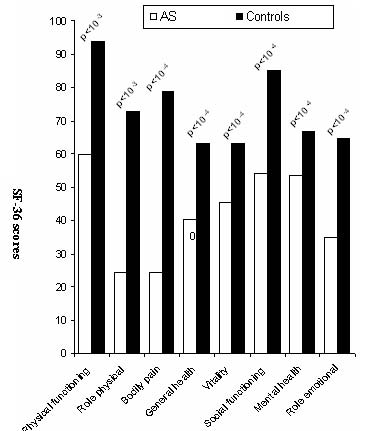
Figure 1A: Comparison of mean SF-36 scores in patients and controls for male (M).
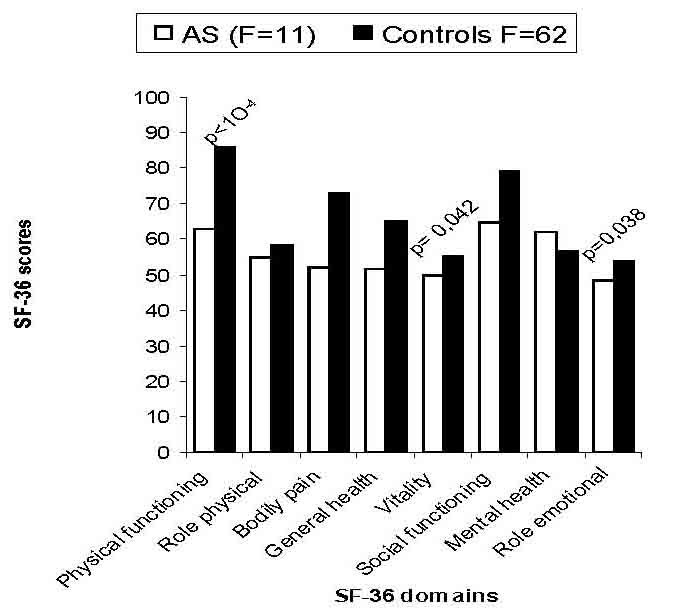
Figure 1B: Comparison of mean SF-36 scores in patients with the general population for female (F).
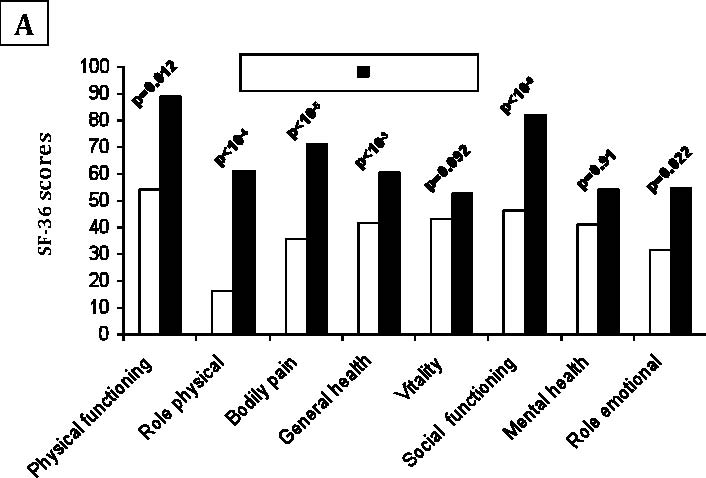
Figure 2A: Comparison of mean SF-36 scales scores in patients with controls in rural regions.
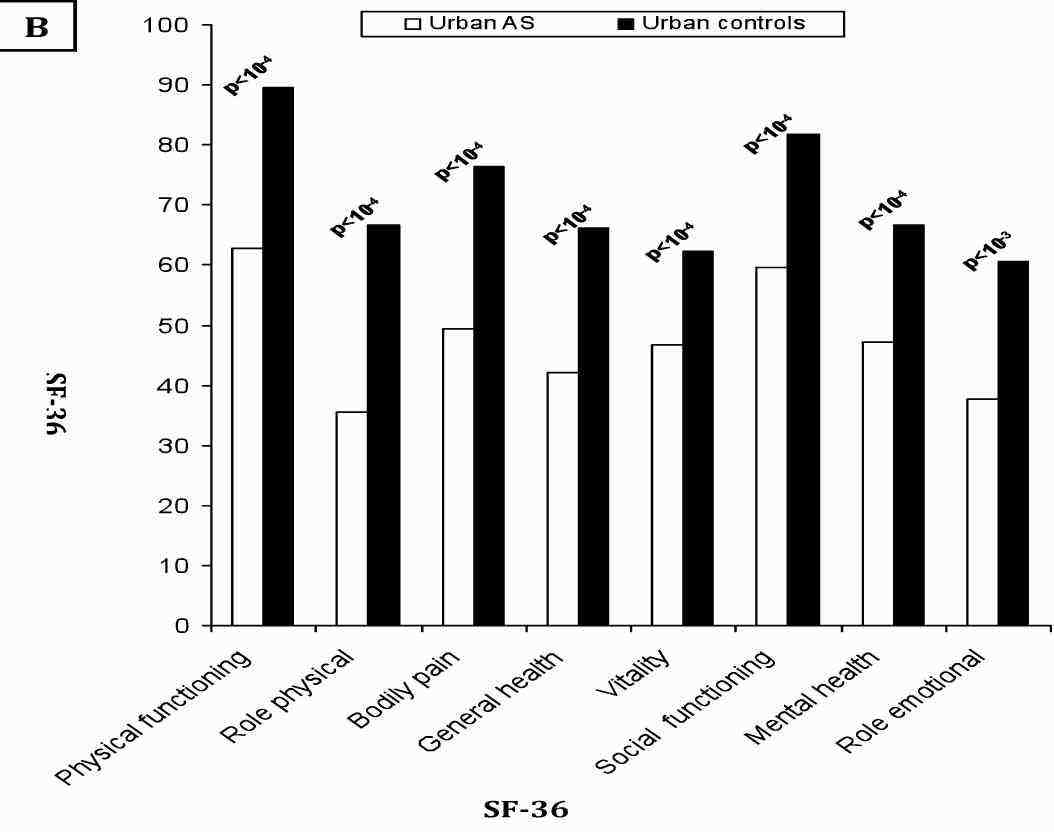
Figure 2B: Comparison of mean SF-36 scales scores in patients with controls in urban regions.
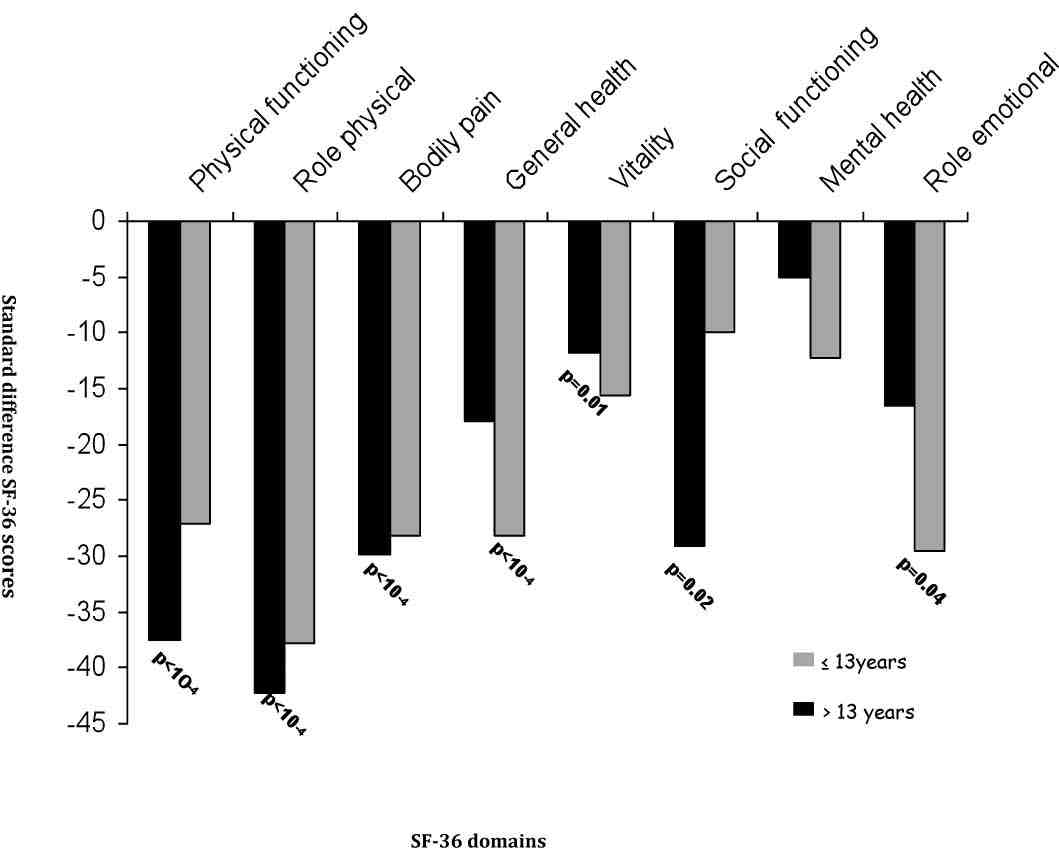
Figure 3: Standard difference scores for high (≤13years) and low (>13 years) education groups.
All SF-36 scales were not correlated with age at the onset of disease and with disease duration. The physical role was significantly higher in patients with high education level than in patients with low education level (p=0.01). Physical functioning was significantly better in employed patients than in those unemployed (p=0.04). Among the disease parameters; family history of AS, progressive onset of AS, peripheral arthritis, hip involvement and ESR level had no influence on the SF-36 scores. Nevertheless, axial and peripheral arthritis VAS were correlated with all scales of the SF-36. Morning stiffness was correlated with bodily pain, vitality and mental health. (Table 2)
All scales of SF-36 were correlated with BASFI, BASDAI and BAS-G, (Table 2). Only physical functioning and general health were correlated with BASMI.
Table 2: Correlations between SF-36 scores in Patients with AS and Disease-specific Instruments.
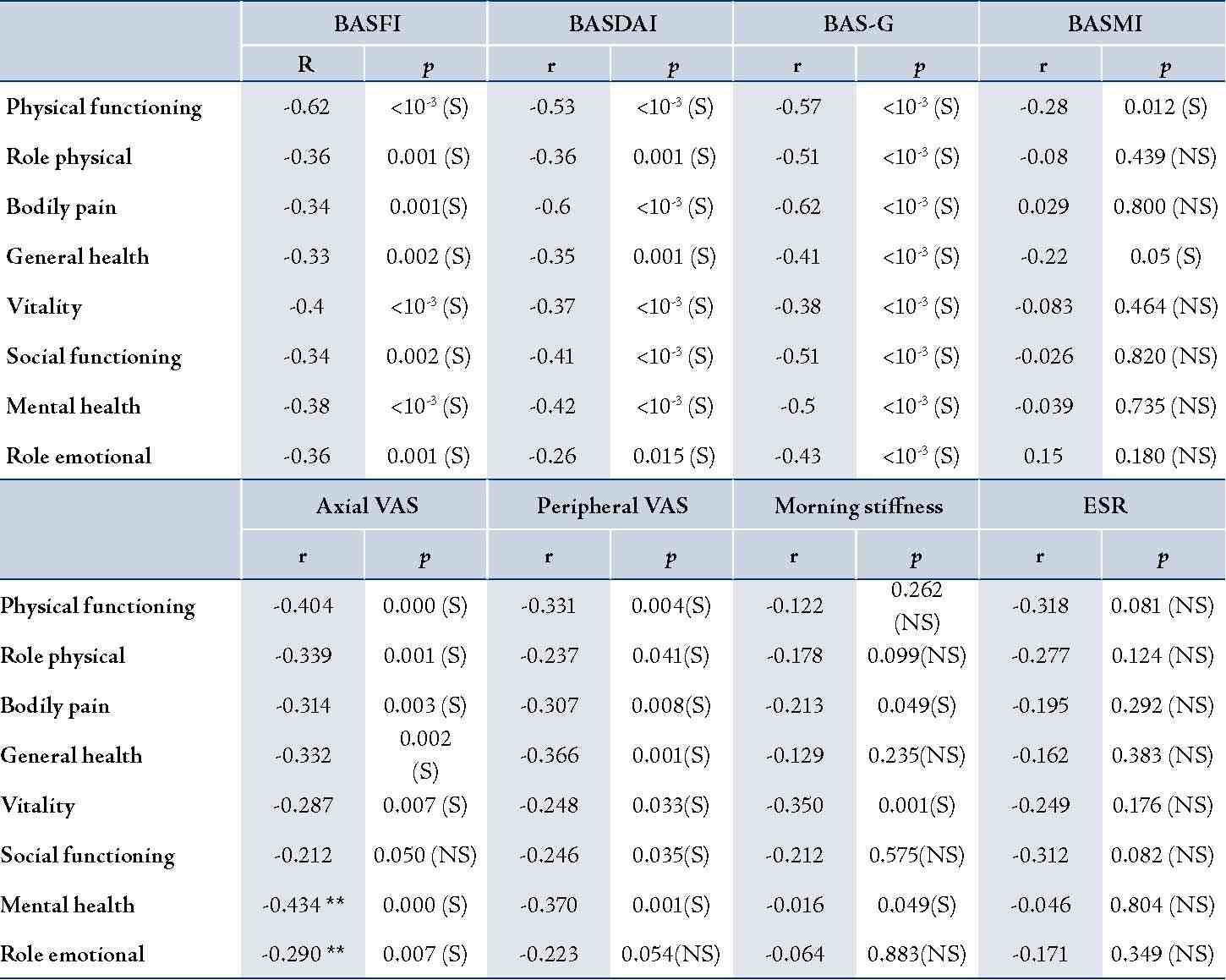
BASFI: Bath Ankylosing Functional Index; BASDAI: Bath Ankylosing Disease Activity Index; BAS-G: Bath Ankylosing Spondylitis Patient Global Score; BASMI: Bath Ankylosing Spondylitis Metrology Index; r: Pearson coefficient; p: signification level; N: number of patients; S: significant; NS: not significant.
Discussion
The results of the present study show great impairment in the quality of life in patients with AS on all scales although some studies reported less affected quality of life in AS patients.18-21 This finding may be explained either by the recruitment mode of patients or by more severe form of AS in North African patients; this hypothesis seems to be affirmed by a study in Moroccan patients.22 The study patients included in this investigation were recruited from the Department of Rheumatology where patients with severe affectation are usually referred.
Concerning the influence of gender on the quality of life, the current study conveyed better quality of life in women than in men. In fact, all male patients with AS reported significantly impaired health on all scales of the SF-36 compared with the general population. Whereas female patients reported poorer health on only three scales (physical functioning, general health, and bodily pain). Differences between men and women regarding course of disease were reported.23-25 In fact, women are supposed to have milder disease course and a later age of onset.20
It is well known that AS may result in physical impairment; however, the present study shows that mental health was more affected than physical health. These results were confirmed by a Moroccan study but not by other studies in Caucasian patients.18-22 (Fig. 4)
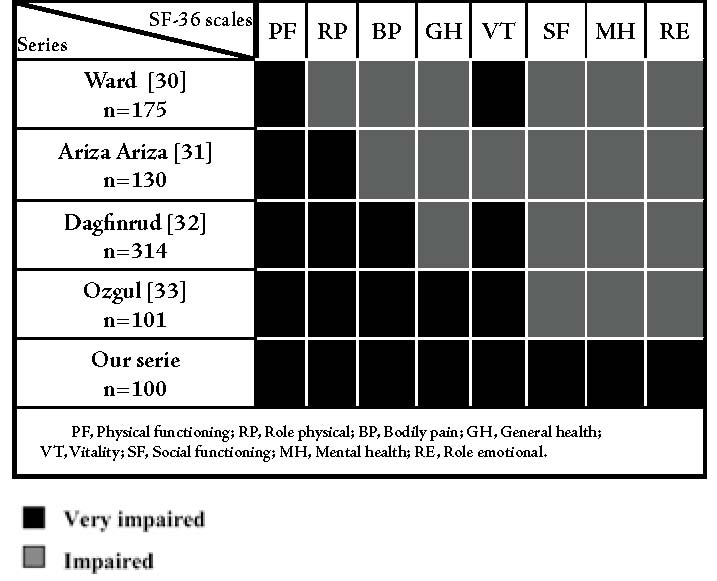
Figure 4: Health-related quality of life in patients with AS (literature data).
This variation could be explained by cultural differences of disease perception in different populations. Comparable with other studies, the results demonstrate more impaired health among the low-educated group wherein mental health seemed to be more affected than physical health.18,20 Few studies were done to evaluate the impact of this affliction on the professional activity of patients. Most of these studies affirm that the rate of work withdrawal due to AS is raised comparing to the general population.26 About a third of the study patients had stopped working indefinitely while a third stopped temporarily because of the disease. Concerning the quality of life of unemployed patients, only the domain of physical activity was shown to be more altered in this study; however, the majority of reports note a worsening quality of life concerning all the items of the SF-36 among unemployed patients.27 These variations can be due to some specific features of every population and the coping capacity of patients. Among the specific disease indicators, high scores of BASDAI, BASFI, BASMI and BASG-s were shown to be associated with worse quality of life both in this study and in the literature.19,22 In fact, disease activity, functional impairment, pain, and mobility limitation indeed have great influence on a patient’s quality of life. These factors may be considered in the management of the patient’s disease. Nevertheless, high disease activity, functional impairment and pain levels were reported in 75% of patients with AS treated by NSAIDs.28,29
It seems that a large proportion of patients with AS may be considered as candidates for new treatments like anti-TNF-α, which stops the inflammation process, delay disease progression, and improve their health-related quality of life.30 In fact, Han et al. while studying the impact of infliximab on the quality of life on patients with AS, and reported that patients who received anti-TNF-α therapy showed significant improvement on physical component summary scores compared with those who received placebo. The magnitude of the difference of improvement (effect size) between infliximab and placebo groups was 10.1 (9.2-11.0) in the AS patients.31
Conclusion
In summary, this study shows increased impairment on the quality of life related to AS, affecting mental health more than physical health. Social and professional factors associated with poor quality of life requires a better and multidisciplinary healthcare for patients with AS. Physical combined with pharmacological treatments, patient education, management of fatigue, protection of the joints, and adaptation of the workstation are some of the methods which could help address disease parameters that are mostly associated with deterioration of quality of life following AS and thus aid in improving the general health of patients.
Acknowledgements
The authors reported no conflict of interest and no funding was received for this work.
References
1. Russell AS. Ankylosing spondylitis: History. In: Klippel JH, Dieppe PA. Rheumatology. 2nd edn. London: Mosby, 1998, pp. 1-2.
2. Zink A, Braun J, Listing J, Wollenhaupt J; German Collaborative Arthritis Centers. Disability and handicap in rheumatoid arthritis and ankylosing spondylitis-results from the German rheumatological database. J Rheumatol 2000 Mar;27(3):613-622.
3. Ward MM. Quality of life in patients with ankylosing spondylitis. Rheum Dis Clin North Am 1998 Nov;24(4):815-827, x.
4. van der Heijde D, van der Linden S, Bellamy N, Calin A, Dougados M, Khan MA. Which domains should be included in a core set for endpoints in ankylosing spondylitis? Introduction to the ankylosing spondylitis module of OMERACT IV. J Rheumatol 1999 Apr;26(4):945-947.
5. Jenkinson C, Coulter A, Wright L. Short form 36 (SF36) health survey questionnaire: normative data for adults of working age. BMJ 1993 May;306(6890):1437-1440.
6. Hemingway H, Stafford M, Stansfeld S, Shipley M, Marmot M. Is the SF-36 a valid measure of change in population health? Results from the Whitehall II Study. BMJ 1997 Nov;315(7118):1273-1279.
7. Moll JM. New criteria for the diagnosis of ankylosing spondylitis. Scand J Rheumatol Suppl 1987;65:12-24.
8. Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992 Jun;30(6):473-483.
9. Tarlov AR, Ware JE Jr, Greenfield S, Nelson EC, Perrin E, Zubkoff M. The Medical Outcomes Study. An application of methods for monitoring the results of medical care. JAMA 1989 Aug;262(7):925-930.
10. Ware JE Jr, Kosinski M, Bayliss MS, McHorney CA, Rogers WH, Raczek A. Comparison of methods for the scoring and statistical analysis of SF-36 health profile and summary measures: summary of results from the Medical Outcomes Study. Med Care 1995 Apr;33(4)(Suppl):AS264-AS279.
11. Ware JE Jr. The SF-36 Health Survey. In: Spilker B, ed. Quality of life and pharmacoeconomics in clinical trials. Philadelphia: Lippincott-Raven, 1996:337-43.
12. Abdulmohsin SA, Coons SJ, Draugalis JR, and al. Translation of the Rand 36-Item Survey 1.0 into Arabic. Rand-Documents 1997;7995:1-33.
13. Coons SJ, Alabdulmohsin SA, Draugalis JR, Hays RD. Reliability of an Arabic version of the RAND-36 Health Survey and its equivalence to the US-English version. Med Care 1998 Mar;36(3):428-432.
14. Garrett S, Jenkinson T, Kennedy LG, Whitelock H, Gaisford P, Calin A. A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J Rheumatol 1994 Dec;21(12):2286-2291.
15. Calin A, Garrett S, Whitelock H, Kennedy LG, O’Hea J, Mallorie P, et al. A new approach to defining functional ability in ankylosing spondylitis: the development of the Bath Ankylosing Spondylitis Functional Index. J Rheumatol 1994 Dec;21(12):2281-2285.
16. Jenkinson TR, Mallorie PA, Whitelock HC, Kennedy LG, Garrett SL, Calin A. Defining spinal mobility in ankylosing spondylitis (AS). The Bath AS Metrology Index. J Rheumatol 1994 Sep;21(9):1694-1698.
17. Jones SD, Steiner A, Garrett SL, Calin A. The Bath Ankylosing Spondylitis patient global score (BAS-G). Br J Rheumatol 1996 Jan;35(1):66-71.
18. Ward MM. Health-related quality of life in ankylosing spondylitis: a survey of 175 patients. Arthritis Care Res 1999 Aug;12(4):247-255.
19. Ariza-Ariza R, Hernández-Cruz B, Navarro-Sarabia F. Physical function and health-related quality of life of Spanish patients with ankylosing spondylitis. Arthritis Rheum 2003 Aug;49(4):483-487.
20. Dagfinrud H, Mengshoel AM, Hagen KB, Loge JH, Kvien TK. Health status of patients with ankylosing spondylitis: a comparison with the general population. Ann Rheum Dis 2004 Dec;63(12):1605-1610.
21. Ozgül A, Peker F, Taskaynatan MA, Tan AK, Dinçer K, Kalyon TA. Effect of ankylosing spondylitis on health-related quality of life and different aspects of social life in young patients. Clin Rheumatol 2006 Mar;25(2):168-174.
22. Ibn Yacoub Y, Amine B, Laatiris A, Abouqal R, Hajjaj-Hassouni N. Health-related quality of life in Moroccan patients with ankylosing spondylitis. Clin Rheumatol 2011 May;30(5):673-677.
23. Will R, Edmunds L, Elswood J, Calin A. Is there sexual inequality in ankylosing spondylitis? A study of 498 women and 1202 men. J Rheumatol 1990 Dec;17(12):1649-1652.
24. Kidd B, Mullee M, Frank A, Cawley M. Disease expression of ankylosing spondylitis in males and females. J Rheumatol 1988 Sep;15(9):1407-1409.
25. Jiménez-Balderas FJ, Mintz G. Ankylosing spondylitis: clinical course in women and men. J Rheumatol 1993 Dec;20(12):2069-2072.
26. Ramos-Remus C, Prieto-Parra RE, Michel-Diaz J, and al. A five-year cumulative analysis of labor-status and lost working days in patients with ankylosing spondylitis (AS). Arthritis Rheum Suppl 1998:1136.
27. Ward MM, Kuzis S. Risk factors for work disability in patients with ankylosing spondylitis. J Rheumatol 2001 Feb;28(2):315-321.
28. Dougados M, Dijkmans B, Khan M, Maksymowych W, van der Linden S, Brandt J. Conventional treatments for ankylosing spondylitis. Ann Rheum Dis 2002 Dec;61(Suppl 3):iii40-iii50.
29. Hermann J, Müller T, Yazdani-Biuki B, Aringer M, Herold M, Ebner W, et al. High disease activity of ankylosing spondylitis in the community before the introduction of tumour necrosis factor blockers. Ann Rheum Dis 2007 Apr;66(4):561-562.
30. Stockdale J, Goodacre L. 'It's magic stuff': The experiences of patients with ankylosing spondylitis taking anti-TNF-alpha medication. Musculoskelet Care 2008;7:162-177 .
31. Han C, Smolen JS, Kavanaugh A, and al. The impact of infliximab treatment on quality of life in patients with inflammatory rheumatic diseases. Arthritis Res Ther 2007;9:103.
|