Urinary tract infection (UTI) is one of the most common health problems and the second most common clinical indication for empirical antibiotic treatment in primary and secondary health care settings, which entails a high consumption of health system resources. It has a 50% to 60% lifetime incidence in adult women with a significant personal, societal, and economic burden.1,2 Due to the frequent use of antibiotics to treat UTI and the increase in global antibiotic resistance in recent years, it is common to find uropathogens with multiple resistance mechanisms. These uropathogens include quinolone resistant bacteria, broad-spectrum β-lactamase producers, and carbapenemase producers. The development of these resistances has limited the use of most available oral front-line antibiotics as empirical therapeutic options for uncomplicated UTI.3
Fosfomycin is an old broad-spectrum bactericidal antibiotic. It was recently included in the drug formulary at our hospital despite being used since 1969. It is an oral derivative of phosphonic acid and is able to inhibit the synthesis of the bacterial cell wall in both gram-positive and gram-negative bacteria through inhibition of the initial step involving phosphoenolpyruvate synthetase with very low toxicity. Its pharmacokinetic profile encourages its use for UTIs. The mean peak urinary concentration of a single oral dose of 3 g fosfomycin tromethamine occurs within four hours. This concentration is sufficient to inhibit most urinary pathogens and is maintained for one to two days.4,5 It is effective against a wide range of resistant uropathogens, including multi-drug resistant Pseudomonas aeruginosa, extended-spectrum β-lactamase (ESBL)-producing bacteria, carbapenem-resistant Enterobacteriaceae (CRE), and vancomycin-resistant Enterococci. It is usually given as a single dose for the treatment of cystitis.6
Fosfomycin trometamol, nitrofurantoin, and pivmecillinam are recommended as first choice antimicrobial therapy in acute uncomplicated cystitis in otherwise healthy women, according to the European Association of Urology and the Infectious Diseases of America guidelines.7,8 Fosfomycin is a more convenient antibiotic due to the single-dose regimen, the in-vitro activity against the resistant organisms, and the minimal propensity for collateral damage. In contrast, nitrofurantoin has an important limitation for use, as it is contraindicated in patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency, which is common in Bahrain. Pivmecillinam is not available in Bahrain.
Cystitis is one of the most commonly encountered types of bacterial infections. Its antibiotic treatment is usually empirical and should be guided by the local resistance profile of the common uropathogens. In the current study, we aimed to determine the antimicrobial susceptibility profile of ESBL-producing Escherichia coli (E. coli) isolated from the urinary samples to fosfomycin and other antibiotics in patients attending Salmaniya Medical Complex, and decrease the need to use intravenous fluids therapy of UTIs caused by ESBL-producing E. coli.
Methods
We conducted a retrospective observational analysis of all ESBL-producing E. coli isolates from clinical urine samples obtained from January 2018 to December 2019 in the Microbiology Section, Pathology Department, Salmaniya Medical Complex, which is the main tertiary care hospital in Bahrain. We retrospectively collected the data of all E. coli urinary isolates and their antibiotic susceptibility from the laboratory records and tabulated the data in Microsoft Excel. We analyzed the predominant E. coli isolates, including sensitive strain, ESBL-producing strains, and carbapenem-resistant E. coli (E. coli CRE). We included all pure growth of E. coli with colony count ≥ 105 CFU/mL. Duplicate samples and mixed infections were excluded.
All urine samples were inoculated into Cysteine Lactose Electrolyte Deficient Medium (Oxoid, Basingstoke, Hampshire, UK) using a calibrated loop of 1 μL in a safety cabinet. Inoculated plates were incubated at 37 °C for 18–24 hours aerobically. Pure growth of bacteria with a colony count of ≥ 105 CFU/mL for midstream urine was considered significant and further identified using matrix assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometer (Bruker Daltonics, Germany). We did not encounter any mixed infection.
The antimicrobial susceptibility testing of all isolates was done by the standard Kirby-Bauer disk diffusion method using commercial disks (Oxoid) according to Clinical and Laboratory Standards Institute9 to determine the bacterial susceptibility to fosfomycin and other oral antibiotics, including co-amoxiclav, ciprofloxacin, trimethoprim/sulfamethoxazole (TMP/SMX), and nitrofurantoin. The turbidity of the suspension was adjusted to the density of a McFarland 0.5 (Marcy-l’Etoile, France) to standardize the inoculum size.9
For the initial ESBL screening, the urine isolates with an inhibition zone size of ≤ 22 mm with ceftriaxone (30 μg) were identified as potential ESBL producers. The conventional double-disc diffusion test with co-amoxiclav, ceftriaxone, and ceftazidime was used to confirm the ESBL production in Enterobacteriaceae strains. E. coli ATCC 25922 and E. coli ATCC 35218 strains were used for the quality control of susceptibility tests.
We also documented the patients’ age, gender, and nationality and stratified the isolates according to the patients’ age: < 1 year, 1–15 years, 16–50 years, and > 50 years. The isolates were also classified according to their sensitivities into sensitive E. coli strains, ESBL-producing E. coli isolates, and E. coli CRE. Additionally, the isolates were classified into isolates from patients with community or hospital-acquired infections. Finally, the trend of antibacterial sensitivity was followed to compare the isolates collected in 2019 to that collected in 2018.
We used TexaSoft, WINKS SDA Software 2011 (6th, Cedar Hill, Texas, USA) to perform the statistical analysis. The percentages and frequencies were computed for different categorical variables, and a cross-tabulation was computed between every two categorical variables. Finally, we used the chi-squared test to determine whether there were significant relationships between every two categorical variables (according to the year of isolates, gender, age, and community versus nosocomial infections). We considered a p-value of < 0.050 as statistically significant. The Secondary Care Research Committee of Salmaniya Medical Complex, Ministry of Health, Bahrain, approved the study. The study had no ethical consideration as it was a retrospective study with no exposure to any patient data.
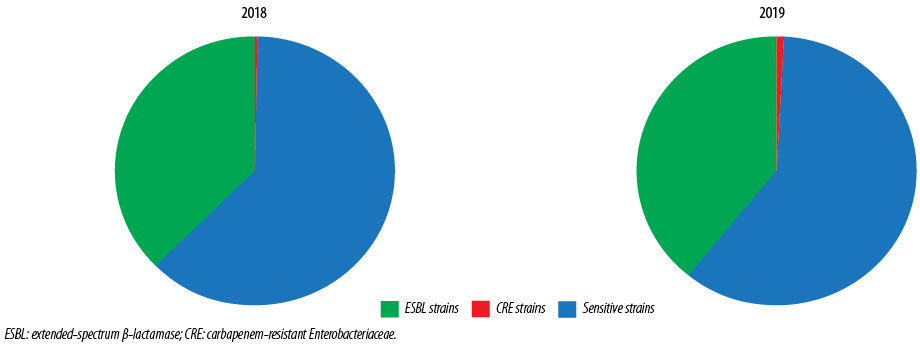
Figure 1: Types of Escherichia coli isolates in 2018 and 2019.
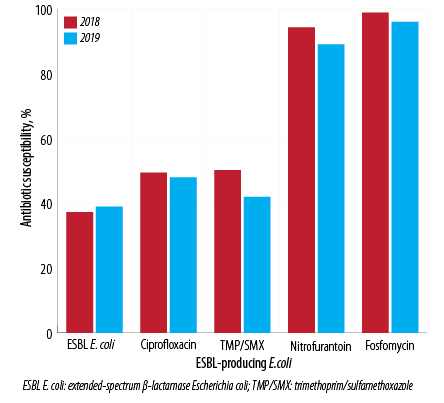
Figure 2: Pattern of antibiotic susceptibility for extended-spectrum β-lactamase-producing Escherichia coli in 2018 and 2019.
Results
The study included 3044 E. coli isolates over the two years, 1539 (50.6%) in 2018 and 1505 (49.4%) in 2019; 38.1% (n = 1161) were ESBL E. coli, and 0.7% (n = 21) were CRE. Samples from females made up 74.8% of isolates. The male to female ratio was 1:3 [Figure 1]. By age stratification, 7.8% of the isolates were observed in infants aged < 1 year, 12.1% in patients between 1–15 years, 37.2% in patients between 16–50 years, and 42.9% in patients > 50 years. The antibiotic susceptibility showed that 50.5% of isolates were sensitive to amoxicillin/clavulanate (which dropped from 54.6% in 2018 to 46.4% in 2019), 72.1% were sensitive to ciprofloxacin (dropped from 73.7% in 2018 to 70.4% in 2019), 2.93% were sensitive to TMP/SMX (dropped from 68.3% in 2018 to 57.4% in 2019), 94.6% were sensitive to nitrofurantoin (dropped from 96.7% in 2018 to 92.5% in 2019), and 99.0% were sensitive to fosfomycin (dropped from 99.5% in 2018 to 98.5% in 2019) [Figure 2].
A total of 1539 E. coli isolates were collected in 2018 [Table 1]. ESBL E. coli isolates formed 37.3% of the total isolates, and E. coli CRE formed 0.3%. Most isolates came from females (74.8%), with a male: female ratio of 1:3.0. However, this ratio increased to 1:2.3 in ESBL strains and 1:1.5 in CRE strains. Most isolates were in the > 50 years age group (42.9%), followed by the 16–50 years (37.2%) age group. Table 1 also showed the percentage of the different strains and their antibiotic susceptibility. Fosfomycin sensitivity was observed in 99.5% of the total isolates and 98.8% of ESBL strains. Additionally, E. coli isolates also showed high susceptibility to nitrofurantoin (96.7% in the total isolates and 94.3% in ESBL strains).
Table 1 also showed the E. coli isolates collected in 2019 with a total of 1505 isolates. ESBL E. coli isolates formed 39.0% of the total isolates (higher than that observed in 2018), and E. coli CRE formed 1.1% (higher than that observed in 2018). Most isolates came from females (74.8%) with a male: female ratio of 1:3.0. However, this ratio increased to 1:2.0 in ESBL strains and 1:1.5 in CRE strains. The age stratifications showed that most isolates were in the age group older than 50 years (42.9%), followed by the 16–50 years age group (37.2%).
Fosfomycin sensitivity was observed to be 98.5% of the total isolates and 96.3% of ESBL strains (slightly lower than observed in 2018) [Table 1]. Additionally, E. coli isolates showed high susceptibility to nitrofurantoin (92.5% in the total isolates and 89.3% in ESBL strains). This was significantly lower than that observed in 2018. Hospital-acquired infection was observed in 10.2% of the total isolates [Table 2]. The percentages in males and the age group below one year were slightly higher in the hospital than in the community. The incidence of ESBL strain was much higher in hospital-acquired UTI at 51.0% compared to 37.6% in community-acquired. Table 2 also showed their antibiotic susceptibility.
Out of 3044 isolates, ESBL E. coli formed 38.1% (1161 isolates) with 49.4% isolated in 2018 and 50.6% isolated in 2019. Table 3 showed the characteristics of ESBL E. coli isolates according to gender and age. Despite most of the isolates coming from female patients (68.6%), the percentage of ESBL isolates from male patients (31.4%) was higher when compared to the percentage of males with sensitive E. coli strains (20.6%). This indicates that males are more likely to have ESBL E. coli strains than females. The over 50s age group had the highest incidence of ESBL E. coli isolates (51.2%), followed by the 16-50 years (30.2%) group. When we compare ESBL to the total number of the isolates in each group, the percentage of ESBL strains were 43.1% in the age group < 1 year, 30.6% for the 1–15 years age group, 30.2% for the age group between 16–50 years, and 46.5% for the age group > 50 years.
Table 1: The different strains of E. coli and its demographic data and antibiotic susceptibility for cases isolated in 2018 and 2019.
|
Total isolates |
1539 (100) |
1505 (100) |
5 (0.3) |
16 (1.1) |
960 (62.4) |
902 (60.0) |
574 (37.3) |
587 (39.0) |
|
Female |
1151 (74.8) |
1133 (75.3) |
3 (60.0) |
6 (37.5) |
746 (77.7) |
733 (81.3) |
402 (70.0) |
394 (67.1) |
|
Male |
388 (25.2) |
372 (24.7) |
2 (40.0) |
10 (62.5) |
214 (22.3) |
169 (18.7) |
172 (30.0) |
193 (32.9) |
|
M/F ratio |
1:3.0 |
1:3.0 |
1:1.5 |
1:0.6 |
1:3.5 |
1:4.3 |
1:2.3 |
1:2.0 |
|
Age, years |
|
< 1 |
130 (8.4) |
118 (7.8) |
0 (0.0) |
1 (6.3) |
81(8.4) |
59 (6.5) |
49 (8.5) |
58 (9.9) |
|
1–15 |
174 (11.3) |
182 (12.1) |
2 (40.0) |
2 (12.5) |
124 (12.9) |
124 (13.7) |
48 (8.4) |
61 (10.4) |
|
16–50 |
603 (39.2) |
560 (37.2) |
1 (20.0) |
3 (18.8) |
415 (43.2) |
379 (42.0) |
187 (32.6) |
164 (27.9) |
|
> 50 |
632 (41.1) |
645 (42.9) |
2(40.0) |
10 (62.5) |
340 (35.4) |
340 (37.7) |
290 (50.5) |
304 (51.8) |
|
Oral Antibiotic Sensitivity |
|
Amox/clav |
841 (54.6) |
698 (46.4) |
0 (0.0) |
0 (0.0) |
847 (88.2) |
698 (77.4) |
0 (0.0) |
0 (0.0) |
|
Ciprofloxacin |
1135 (73.7) |
1060 (70.4) |
1 (20.0) |
0 (0.0) |
850 (88.5) |
855 (95) |
284 (49.5) |
284 (48.4) |
|
TMP/SMX |
1051 (68.3) |
864 (57.4) |
1 (20.0) |
2 (12.5) |
762 (79.4) |
517 (57.3) |
288 (50.2) |
246 (41.9) |
|
Nitrofurantoin |
1488 (96.7) |
1392 (92.5) |
4 (80.0) |
13 (81.3) |
943 (98.2) |
767 (85.0) |
541 (94.3) |
524 (89.3) |
E. coli: Escherichia coli; CRE: carbapenem-resistant Enterobacteriaceae; E. coli: Escherichia coli; ESBL: extended-spectrum β-lactamase; M/F: male/female; Amox/clav: amoxicillin/clavulanate; TMP/SMX: trimethoprim/sulfamethoxazole.
Table 2: Comparison between demographics, types of E. coli isolates, and antibiotic susceptibility between community- and hospital-acquired UTI caused by E. coli in 2019.
|
Isolates |
1352 (89.8) |
153 (10.2) |
|
Female |
1022 (75.6) |
111 (72.5) |
|
Male |
330 (24.4) |
42 (27.5) |
|
M/F ratio |
1:3.1 |
1:2.6 |
|
Age, years |
|
< 1 |
100 (7.4) |
18 (11.8) |
|
1–15 |
171 (12.6) |
11 (7.2) |
|
16–50 |
537 (39.7) |
49 (32.0) |
|
> 50 |
544 (40.2) |
75 (49.0) |
|
Nationality |
|
Bahraini |
1140 (84.3) |
123 (804) |
|
Non-Bahraini |
212 (15.7) |
30 (19.6) |
|
Sensitive E. coli strains |
836 (61.8) |
66 (43.1) |
|
ESBL producing strains |
509 (37.6) |
78 (51.0) |
|
E. coli CRE |
7 (0.5) |
9 (5.9) |
|
Oral antibiotic Susceptibility |
|
Amox/clav |
651 (48.2) |
47 (30.7) |
|
Ciprofloxacin |
975 (72.1) |
86 (56.2) |
|
TMP/SMX |
792 (58.6) |
73 (47.7) |
|
Nitrofurantoin |
1261 (93.3) |
132 (86.3) |
UTI: urinary tract infection; CRE: carbapenem-resistant Enterobacteriaceae; E. coli: Escherichia coli; ESBL: extended-spectrum β-lactamase; M/F: male/ female; Amox/clav: amoxicillin/clavulanate; TMP/SMX: trimethoprim/sulfamethoxazole.
Table 3: ESBL Escherichia coli isolates antibiotic susceptibility according to gender and age group.
|
Total ESBL (n = 1161) |
365 (31.4) |
796 (68.6) |
107 (9.2) |
109 (9.3) |
351 (30.2) |
594 (51.2) |
|
ESBL in 2018 |
172 (47.1) |
402 (50.5) |
49 (45.8) |
48 (44) |
187 (53.2) |
290 (48.8) |
|
ESBL in 2019 |
193 (52.9) |
394 (49.5) |
58 (54.2) |
61 (56) |
164 (46.7) |
304 (51.2) |
|
Susceptibility to ciprofloxacin % |
184 (50.4) |
384 (48.2) |
67 (62.6) |
27 (38.6) |
165 (43.1) |
309 (51.4) |
|
Susceptibility to TMP/SMX % |
175 (47.9) |
359 (45.1) |
50 (46.7) |
15 (21.4) |
162 (42.3) |
307 (51.1) |
|
Susceptibility to nitrofurantoin % |
325 (89.0) |
740 (93.0) |
76 (71.0) |
70(100) |
370 (96.6) |
549 (91.3) |
ESBL: extended-spectrum β-lactamase, TMP/SMX: trimethoprim/sulfamethoxazole.
The antibiotic susceptibility of ESBL E. coli during the study showed susceptibility to TMP/SMX in 46.1% of the isolates (50.2% in 2018 dropped to 41.9% in 2018), to ciprofloxacin in 49.0% of isolates (49.5% in 2018 dropped to 48.4% in 2019), to nitrofurantoin in 94.0% of isolates (94.3% in 2018 dropped to 89.3% in 2019), and fosfomycin in 97.6% of isolates (98.8% in 2018 dropped to 96.3% in 2019). ESBL E. coli isolates from the females showed a higher susceptibility to nitrofurantoin (93.0%) and fosfomycin (97.7%) compared to the isolates from males, which showed 89.0% susceptibility to nitrofurantoin and 97.0% to fosfomycin. On the other hand, ESBL E. coli isolates from males showed higher susceptibility to TMP/SMX (47.9%) and ciprofloxacin (50.4%) than observed in ESBL E. coli isolates from females (45.1% to TMP/SMX and 48.2% to ciprofloxacin). The ESBL isolates derived from infants < 1 year showed the least susceptibility to fosfomycin (78.5%) while it keeps being high (around 99%) in the other age groups. The same also was observed for nitrofurantoin, which had the lowest susceptibility in infants under one year (71.0%). In contrast, > 90.0% of the ESBL isolates were susceptible to nitrofurantoin in the other age groups. TMP/SMX had the lowest susceptibility among all the antibiotics, as low as 21.4% in the age group between 1–15 years. Ciprofloxacin showed moderate susceptibility [Table 3].
Discussion
ESBL producing organisms are important causes of drug-resistant UTIs. ESBLs are enzymes capable of hydrolyzing penicillins, broad-spectrum cephalosporins, and monobactams.10 An effective oral antibiotic against resistant strains of E. coli can reduce the need for parenteral antibiotics, and hence the need for hospitalization. In the current study, we observed the presence of high susceptibility of ESBL strains to nitrofurantoin and fosfomycin, and less extent to TMP/SMX and ciprofloxacin. According to the newborn screening test, the rate of G6PD deficiency is very high in Bahrain (up to 18% in males and 10% in females).11 Nitrofurantoin, TMP/SMX, and ciprofloxacin are not safe for patients with G6PD deficiency and are better avoided as they can trigger hemolytic crises in people with G6PD deficiency. For this reason, fosfomycin is an ideal oral antibiotic and more suitable than the other three antibiotics included in our study. Fosfomycin was recently introduced into Bahrain drug formulary. The high susceptibility of E. coli to fosfomycin we observed in this study is comparable to the work of Maraki et al,5 from Greece, who observed that all E. coli isolates in their study were susceptible to fosfomycin. Similarly, Fajfr et al,12 from the Czechia, Ouzdi et al,13 from Morocco, and Plate et al,14 from Switzerland found high susceptibility of ESBL E. coli urinary isolates to fosfomycin (95.8%, 96%, and 99.4%, respectively). This worldwide low rate of ESBL E. coli resistance to fosfomycin may be related to the mechanism of resistance development, which is chromosomal encoded and not plasmid dependent.15
However, the rate of susceptibility to fosfomycin was relatively lower in ESBL isolates from Korea and Israel (93.7%, and 69%, respectively).16,17
We found that the rates of ESBL E. coli are affected by gender and age. We observed an increase in the percentage of ESBL in males (31.4% of total ESBL isolates compared to 20.6% of total E. coli isolates). At the same time, the rate of fosfomycin susceptibility in ESBL E. coli isolates derived from females was relatively higher (97.7%) than those observed in isolates derived from males (97.0%). This also was observed in another study, which observed that males are more likely to have more resistance to fosfomycin than females, as UTI in males is usually complicated with more chronicity and more frequent exposure to antibiotic therapies. However, their study was designed for uncomplicated UTI and had fewer isolates than our study.18 Despite being more frequent in females than in males, UTIs in men are often complicated due to the higher chance of having malformation and renal involvement and thus require more attention and meticulous investigations.19 As the male gender is considered a risk factor for complicated UTIs, this may explain the relatively high ESBL rate in males. In our study, despite ESBL E. coli found in 9.2% of infants aged < 1 year, this age group had the lowest susceptibility rate to fosfomycin among ESBL E. coli isolates compared to all other age groups (78.5%). E. coli is one of the most common bacterial causes of early neonatal sepsis. Unfortunately, there are very little data on bacteremia caused by multidrug-resistant Gram-negative bacilli in the pediatric age. In a recent Italian study conducted as a part of the Antibiotic Resistance and Prescribing in European Children Project, which analyzed > 1000 episodes of bacteremia, 26% of episodes were caused by Gram-negative microorganisms, 39% of which were multidrug-resistant.20 We do not have an explanation for the relative decrease in fosfomycin susceptibility in ESBL E. coli in infants < 1 year of age. A possible explanation is the transmission of these resistant strains from the mothers to their infants, which needs to be proved in future research, as fosfomycin is not used in our facility to treat young children due to lack of availability of liquid form.
We observed a marked increase in ESBL and E. coli CRE isolates among hospital-acquired (51.0% and 5.9%, respectively) compared to the community-acquired (37.6% and 0.5%, respectively) UTIs observed in 2019. A similar finding from Kuwait was observed; ESBL was about 12% in community-acquired and 26% in hospital-acquired UTI.21 Castillo-Tokumori et al,22 found that a history of previous hospitalization, surgery, and antibiotics are risk factors for ESBL E. coli in community-acquired UTI and should be considered when treating this type of infection. At the same time, Lee et al,23 found that a history of prior UTI within one year and underlying cerebrovascular disease are independent risk factors for the acquisition of ESBL-producing E. coli. The percentage of ESBL E. coli increased from 37.3% in 2018 to 39.0% in 2019, and the susceptibility to fosfomycin decreased from 98.8% in 2018 to 96.3% in 2019. This emphasizes the need to follow a strict antimicrobial stewardship guideline. Further studies are needed to evaluate the mechanism of resistance of E. coli and the other microorganisms to fosfomycin.
Our study has some limitations. It was a retrospective study, and we did not do genetic testing for ESBL strain detected. We also did not correlate the clinical outcome with the laboratory resistance pattern.
Conclusion
ESBL E. coli is an important cause of UTI in Bahrain. Fosfomycin is a very effective oral antimicrobial. It still retains a high efficacy against ESBL E. coli, which helps to decrease the need for parenteral therapy and hospitalization. Judicious use of this promising antimicrobial is needed to avoid increasing the rate of microbial resistance to it.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study. The investigation was carried out in accordance with the latest version of the Declaration of Helsinki and was approved by the Research and Ethics Committee at the Ministry of Health, Bahrain. No consent was collected as this an observational study.
Acknowledgements
We thank the anonymous referees for their useful suggestions.
references
- 1. Shill MC, Huda NH, Moain FB, Karmakar UK. Prevalence of uropathogens in diabetic patients and their corresponding resistance pattern: results of a survey conducted at diagnostic centers in Dhaka, Bangladesh. Oman Med J 2010 Oct;25(4):282-285.
- 2. Medina M, Castillo-Pino E. An introduction to the epidemiology and burden of urinary tract infections. Ther Adv Urol 2019 May;11:1756287219832172.
- 3. Liu HY, Lin HC, Lin YC, Yu SH, Wu WH, Lee YJ. Antimicrobial susceptibilities of urinary extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae to fosfomycin and nitrofurantoin in a teaching hospital in Taiwan. J Microbiol Immunol Infect 2011 Oct;44(5):364-368.
- 4. Dijkmans AC, Zacarías NV, Burggraaf J, Mouton JW, Wilms EB, van Nieuwkoop C, et al. Fosfomycin: pharmacological, clinical and future perspectives. Antibiotics (Basel) 2017 Oct;6(4):24.
- 5. Maraki S, Samonis G, Rafailidis PI, Vouloumanou EK, Mavromanolakis E, Falagas ME. Susceptibility of urinary tract bacteria to fosfomycin. Antimicrob Agents Chemother 2009 Oct;53(10):4508-4510.
- 6. Neuner EA, Sekeres J, Hall GS, van Duin D. Experience with fosfomycin for treatment of urinary tract infections due to multidrug-resistant organisms. Antimicrob Agents Chemother 2012 Nov;56(11):5744-5748.
- 7. Guidelines EA. Edn. Presented at the EAU Annual Congress Amsterdam the Netherlands. 2020 [cited 2020 June 23]. Available from: https://uroweb.org/guideline/urological-infections/#6.
- 8. Gupta K, Hooton TM, Naber KG, Wullt B, Colgan R, Miller LG, et al; Infectious Diseases Society of America; European Society for Microbiology and Infectious Diseases. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the infectious diseases society of America and the European society for microbiology and infectious diseases. Clin Infect Dis 2011 Mar;52(5):e103-e120.
- 9. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing, 30th ed. Melvin P. Weinstein: clinical and laboratory standards institute. 2020 [2020 August 30]. Available from: https://clsi.org/standards/products/microbiology/documents/m100/.
- 10. Ebrahim-Saraie HS, Nezhad NZ, Heidari H, Motamedifar A, Motamedifar M. Detection of antimicrobial susceptibility and integrons among extended-spectrum β-lactamase producing uropathogenic Escherichia coli isolates in Southwestern Iran. Oman Med J 2018 May;33(3):218-223.
- 11. Al-Arayyed S, Hamza AA, Sultan B, et al. Neonatal screening for genetic blood diseases. Bah Med Bull 2007; 29(3):88-90.
- 12. Fajfr M, Louda M, Paterová P, Ryšková L, Pacovský J, Košina J, et al. The susceptibility to fosfomycin of Gram-negative bacteria isolates from urinary tract infection in the Czech Republic: data from a unicentric study. BMC Urol 2017 Apr;17(1):33.
- 13. Ouzdi ZA, Arsalane L, El Kamouni Y, Zouhair S. The resistance of uropathogenic bacteria to fosfomycin. Pathology and Laboratory Medicine. 2018;2(2):47-50.
- 14. Plate A, Kronenberg A, Risch M, Mueller Y, Di Gangi S, Rosemann T, et al. Active surveillance of antibiotic resistance patterns in urinary tract infections in primary care in Switzerland. Infection 2019 Dec;47(6):1027-1035.
- 15. Cattoir V, Guérin F. How is fosfomycin resistance developed in Escherichia coli? Future Microbiol 2018 Dec;13:1693-1696.
- 16. Seok H, Choi JY, Wi YM, Park DW, Peck KR, Ko KS. Fosfomycin resistance in Escherichia coli isolates from South Korea and in vitro Activity of Fosfomycin alone and in combination with other antibiotics. Antibiotics (Basel) 2020 Mar;9(3):112.
- 17. Peretz A, Naamneh B, Tkhawkho L, Nitzan O. High rates of fosfomycin resistance in gram-negative urinary isolates from Israel. Microb Drug Resist 2019 Apr;25(3):408-412.
- 18. Guneysel O, Suman E, Ozturk TC. Trimethoprim-sulfamethoxazole resistance and fosfomycin susceptibility rates in uncomplicated urinary tract infections: time to change the antimicrobial preferences. Acta Clin Croat 2016 Mar;55(1):49-57.
- 19. Paul R. State of the globe: rising antimicrobial resistance of pathogens in urinary tract infection. J Glob Infect Dis 2018 Jul-Sep;10(3):117-118.
- 20. Folgori L, Livadiotti S, Carletti M, Bielicki J, Pontrelli G, Ciofi Degli Atti ML, et al. Epidemiology and clinical outcomes of multidrug-resistant, gram-negative bloodstream infections in a European tertiary pediatric hospital during a 12-month period. Pediatr Infect Dis J 2014 Sep;33(9):929-932.
- 21. Al Benwan K, Al Sweih N, Rotimi VO. Etiology and antibiotic susceptibility patterns of community- and hospital-acquired urinary tract infections in a general hospital in Kuwait. Med Princ Pract 2010;19(6):440-446.
- 22. Castillo-Tokumori F, Irey-Salgado C, Málaga G. Worrisome high frequency of extended-spectrum beta-lactamase-producing Escherichia coli in community-acquired urinary tract infections: a case-control study. Int J Infect Dis 2017 Feb;55:16-19.
- 23. Lee H, Han SB, Kim JH, Kang S, Durey A. Risk factors of urinary tract infection caused by extended spectrum β-lactamase-producing Escherichia coli in emergency department. Am J Emerg Med 2018 Sep;36(9):1608-1612.