On 11 March 2020, the World Health Organization declared the rapidly spreading outbreak due to the newly emerging severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) as a global pandemic and disease caused by it named coronavirus disease 2019 (COVID-19).1 Healthcare workers (HCWs) are at the front line of outbreak responses and are particularly at risk of being infected from either occupational exposure or infection from the surrounding community. Furthermore, the infected workers can subsequently transmit the virus to other people in healthcare settings or their households and communities.2
Serological response to COVID-19 is still an area for further research as different studies have shown variable results in terms of factors associated with seropositivity, detectable antibodies, duration of positivity, and protection against re-infection. Significant seropositivity with certain specific symptoms compared to other symptoms has been reported.3,4 Further, differences in seropositivity to SARS-CoV-2 between different races and ethnic groups have also been reported.5 The duration of detectable antibodies in the sera of infected people is yet to be determined. However, studies from the Middle East respiratory syndrome coronavirus have shown that antibodies remained detectable for up to one year post-infection.6
In this study, we present data of HCWs with positive COVID infection from one of the main tertiary hospitals that provide care for patients with COVID-19 in Oman. The aim was to identify the source of infection among HCWs (hospital or community-acquired), categories of the infected HCWs, their common symptoms, degree of severity, and their outcome. In addition, the immunoglobulin G (IgG) response to COVID-19 infection for this population was also assessed and presented to determine any significant factors that might affect the seropositivity to SARS-CoV-2 infection.
Methods
In this combined retrospective and prospective (amidirectional) cohort study, we collected data of all HCWs who tested positive for COVID-19 in Al-Nahdha Hospital, Muscat, Oman between 2 April and 24 July 2020. Data were collected timely through direct interview or telephonic communications, when a positive polymerase chain reaction (PCR)test was released by the laboratory to minimize recall bias. Additional information were obtained from the hospital electronic database (Al-Shifa 3 Plus System, locally designed by the Ministry of Health, Oman). Finally, data were transferred to Excel spreadsheet for analysis.
A reverse transcriptase PCR (RT-PCR) test was used to identify HCWs with positive COVID-19 disease. The majority were tested using the GeneXpert® system (Cepheid, USA), while few were tested using Sansure Nucleic Acid Diagnostic Kit (Sansure Biotech, China). Collected variables included age, gender, nationality, presenting symptoms, working area in the hospital, adherence to infection prevention and control measures (yes, no), possible source of infection (hospital vs. community), and severity of illness (mild, moderate, severe) in addition to other variables shown in Table 1. COVID-19 infection among HCWs was considered as hospital-acquired if the infected person had contact with a confirmed COVID-19 case in a hospital setting with no contact in the community, while a community-acquired infection was considered if the infected HCW was in contact with a confirmed COVID-19 case in a community setting. An unknown source was considered if there was no evidence of exposure in the hospital or community. Serum samples were collected from HCWs from 10 August to 2 September 2020, except for two staff whose samples were stored from July. All samples were processed together to detect IgG serological response using enzyme-linked immunosorbent assay (Architect i 1000 SR system, Abbott, USA). Samples that were not processed timely were stored at -20 oC immediately until the subsequent processing time.
Table 1: Characteristics of COVID-19 infected healthcare workers (HCWs) including immunoglobulin G (IgG) response.
|
Age, years |
0.098 |
|
20–29 |
20 (19.4) |
8 (40.0) |
4 (20.0) |
8 (40.0) |
|
|
30–39 |
44 (42.7) |
28 (63.6) |
3 (6.8) |
13 (29.5) |
|
|
40–49 |
28 (27.2) |
15 (53.6) |
7 (25.0) |
6 (21.4) |
|
|
50–59 |
9 (8.7) |
7 (77.8) |
0 (0.0) |
2 (22.2) |
|
|
> 60 |
2 (1.9) |
2 (100) |
0 (0.0) |
0 (0.0) |
|
|
Gender |
0.262 |
|
Male |
41 (39.8) |
25 (61.0) |
4 (9.8) |
12 (29.3) |
|
|
Female |
62 (60.2) |
35 (56.5) |
10 (16.1) |
17 (27.4) |
|
|
Profession |
0.670 |
|
Doctors |
18 (17.5) |
10 (55.6) |
2 (11.1) |
6 (33.3) |
|
|
Nurses |
47 (45.6) |
27 (57.4) |
7 (14.9) |
13 (27.7) |
|
|
Others |
38 (36.9) |
23 (60.5) |
5 (13.2) |
10 (26.3) |
|
|
Symptoms |
|
|
|
|
|
|
Fever |
67 (65.0) |
40 (59.7) |
7 (10.4) |
20 (29.9) |
0.243 |
|
Cough |
55 (53.4) |
32 (58.2) |
8 (14.5) |
15 (27.3) |
0.797 |
|
Sore throat |
51 (49.5) |
30 (58.8) |
7 (13.7) |
14 (27.5) |
1.000 |
|
Body ache |
44 (42.7) |
22 (50.0) |
6 (13.6) |
16 (36.4) |
0.667 |
|
Headache |
17 (16.5) |
8 (47.1) |
3 (17.6) |
6 (35.3) |
0.443 |
|
Anosmia |
7 (6.8) |
4 (57.1) |
1 (14.3) |
2 (28.6) |
0.949 |
|
Ageusia |
2 (1.9) |
1 (50.0) |
0 (0.0) |
1 (50.0) |
0.627 |
|
Diarrhea |
4 (3.9) |
2 (50.0) |
2 (50.0) |
0 (0.0) |
0.103 |
|
Vomiting |
3 (2.9) |
2 (66.7) |
1 (33.3) |
0 (0.0) |
0.515 |
|
SOB* |
4 (3.9) |
2 (50.0) |
0 (0.0) |
2 (50.0) |
0.489 |
|
Flu-like symptoms |
15 (14.6) |
|
0 (0.0) |
|
|
|
Asymptomatic |
1 (1.0) |
1 (100) |
0 (0.0) |
0 (0.0) |
|
|
Outcome |
- |
|
Recovered |
99 (96.1) |
57 (57.6) |
14 (14.1) |
28 (28.3) |
|
|
Admitted |
4 (3.9) |
3 (75.0) |
0 (0.0) |
1 (25.0) |
|
|
Daily contact with patients |
0.118 |
|
Yes |
79 (76.7) |
44 (55.7) |
13 (16.5) |
22 (27.8) |
|
|
No |
24 (23.3) |
16 (66.7) |
1 (4.2) |
7 (29.2) |
|
|
Working in COVID-19 area |
0.026 |
|
Yes |
42 (40.8) |
28 (66.7) |
2 (4.8) |
12 (28.6) |
|
|
No |
61 (59.2) |
32 (52.5) |
12 (19.7) |
17 (27.9) |
|
|
Nationality |
0.008 |
|
Omani |
61 (59.2) |
28 (45.9) |
12 (19.7) |
21 (34.4) |
|
Data given as n (%); SOB: shortness of breath.
The SARS-CoV-2 IgG assay is a qualitative chemiluminescent microparticle immunoassay that detects IgG antibodies to the nucleocapsid protein of SARS-CoV-2 in human serum and plasma. It uses the Abbott Architect i system and reports an index of signal to cut-off (S/C) where the cut-off point for a positive test is ≥ 1.40. According to the manufacturer, the sensitivity of this test is 100% and the specificity is 99.6%.
Data were analyzed using SPSS Statistics (SPSS for Windows, Version 22.0. Chicago, SPSS Inc.). Categorical data including IgG positivity rate and comparison of proportions of positive IgG with age, sex, symptoms, time to serology testing (> or < 2 months), and cycle threshold (Ct) levels (> or < 30) were compared using the chi-square test. The measure of association between different symptoms was also assessed using the chi-square test. Linear relationship and statistically significant differences between IgG level and different independent variables (age, Ct for N2 (nucleocapsid) and envelope (E) gene values) were determined by Pearson’s correlation, two-tailed t-test, and analysis of variance, respectively, to obtain odds ratios (ORs), 95% confidence intervals (95% CIs), and p-values. Fisher’s exact test was used to find an association between symptoms, different age groups, and between different professions and sources of infection. A p-value of < 0.050 was considered to be statistically significant.
Written informed consent was obtained from all HCWs studied before each sample collection. The study was approved by the Research Committee in the Directorate General of Health Services of Muscat Governorate in the Ministry of Health (Identification code: 23843 dated
30 August 2020).
Results
From a total of 974 HCWs in Al Nahdha hospital, 540 (55.4%) were tested for SARS-CoV-2. Of those, 103 (19.1%) tested positive by RT-PCR test, which constitutes 10.6% of the total HCWs in the hospital. The mean age of HCWs positive for COVID-19 was 38.0 years (range = 25–64 years), with 60.2% being females [Table 1]. Nurses were the main affected group (45.6%), followed by doctors (17.5%), with other categories of HCWs being in smaller percentages. From the total infected HCWs, 79 (76.7%) were in daily contact with patients, of which 40.8% were working in
COVID-19 areas.
The most common symptoms were fever (65.0%), followed by cough (53.4%), sore throat (49.5%), and body ache (42.7%) [Table 1]. Loss of smell and taste were found in few cases. Fever was significantly more prevalent in HCWs aged ≥ 40 years (p = 0.033).
Only 3.9% of HCWs required admission, with one patient requiring admission to an intensive care unit. There was no mortality among the studied group. The possible source of infection of SARS-CoV-2 in 50.0% of cases was exposure within the healthcare facility, 38.0% in the community, and in 12.0%, the source of infection could not be determined. Working in COVID-19 areas was more likely to be associated with hospital-acquired than community-acquired infection (p < 0.005). Nurses were more likely to have a hospital-acquired COVID-19 infection than other categories (OR = 4.63, 95% CI: 1.71–12.52, p = 0.002). The most common source of hospital-acquired infection was from colleagues eating together in groups or mixing in changing rooms (94.0%), while (6.0%) were from direct contact with patients. There was no significant difference in the infection source between different age groups or between genders (p = 0.280 and 0.200, respectively).
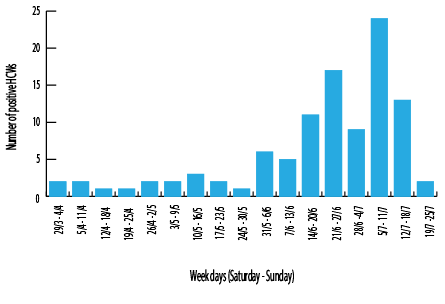
Figure 1: Distribution of COVID-19 positive healthcare workers (HCWs) per week during the study period.
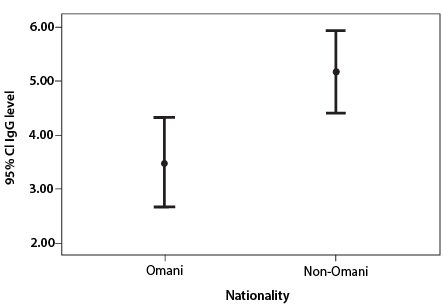
Figure 2: Effect of ethnicity on immunoglobulin G (IgG) level. The lines inside of the box indicate 95% confidence interval (CI) range for the mean value (circled) of each nationality.
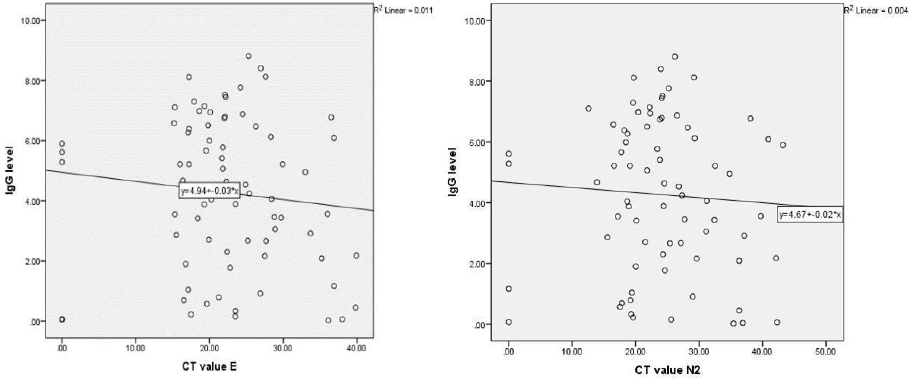
Figure 3: (a) Shows correlation between immunoglobulin G (IgG) level and cycle threshold (Ct) value (E). Two-tailed t-test and Pearson's correlation were used to determine the significance. There is no significant relationship between IgG level and Ct value for E, r(69) = -0.160, p = 0.184. (b) Shows correlation between IgG level and Ct value (N2). Two-tailed t-test and Pearson's correlation were used to determine the significance. There is no significant relationship between IgG level and Ct value for N2, r(70) = -0.116, p = 0.330.
The highest infection rate was noted between 5–11 July 2020 [Figure 1]. The main source in this week was the community (46.0%), and the rest were either healthcare-associated (29.0%) or an unknown source (25.0%).
From the total of 103 HCWs, 71.8% (n = 74) were tested for SARS-CoV-2 IgG. From those, 81.1% (n = 60) tested positive and 18.9% (n = 14) tested negative. The elapsed time between positive PCR and serum sample collection ranged from three weeks to 20 weeks with 50.0% less than eight weeks. Working in COVID-19 areas and being a non-Omani national was significantly associated with positive IgG test (p = 0.026 and 0.008, respectively). Median IgG level (4.58 S/C) was significantly higher in non-Omani nationals (p = 0.004)
[Figure 2]. There was no significant correlation between seropositivity of IgG with gender (p = 0.260), age (p = 0.098, linear regression R2 = 0.025), time to testing (p = 0.550), symptoms (p-value range 0.100–1.000), and Ct value (p-value for Ct E = 1.09 and for N2 = 0.09) [Figure 3]. There was no statistical significance observed in those with daily contact with patients (p = 0.118) or with source of infection (p = 0.491).
Discussion
To the best of our knowledge, this is the first study from Oman that documents the serological response of HCWs to COVID-19 infection and their characteristics. HCWs are the frontline in the battle against COVID-19; therefore, it is important to know their serological response and be aware of how they can be protected from re-infection with the repeated exposure as they continue their clinical duties.
The rate of infection among HCWs in our study was higher over four months (10.6%) than that reported in a study from another local hospital (4.3%)7 and two Dutch hospitals (1%),8 but lower than rates reported from a large Spanish hospital (11.6%) over one month.4 Like many other studies, the rate of infection among female staff was higher than their male counterparts (60.2%), yet it was similar to what was reported by several other studies.7–9 This was in sharp contrast to what was reported from the community, where the rate of infection was higher in males.10 Nurses, in particular, were at a higher risk of acquiring COVID-19 infection from healthcare settings compared to other professions. This was similar to what was reported by Al Maskari et al,7 (38%) and Garcia-Basteiro,et al,4 (48%). This can be attributed to their closer and prolonged contact with COVID-19 infected patients compared to other professions.2 In addition to this fact, the majority of the nurses in many healthcare settings are female. This also explains the higher rate of infection in this group.11
The reported symptoms among HCWs were similar to that reported in other hospitals4,7,9 with fever, cough, and other respiratory symptoms the most common presentations. The majority (96.1%) of HCWs had mild illness with uneventful recovery. No mortality was noted among HCWs in this study; however, other studies reported mortalities, especially in those aged > 65 years old or HCWs with comorbidities.9
In our study, 50.0% of infections among HCWs were attributed to exposure to individuals (a colleague or a patient) within a healthcare setting. A similar rate was reported from the USA (55%),9 while lower rates were documented from a local hospital in Oman (25%).7 Despite regular reinforcement of infection control measures (social distancing and universal masking), higher rates of hospital-acquired COVID-19 infections were mainly due to staff gathering with colleagues (94.0%) during break time at the beginning of the pandemic, as per the information provided by the participants. Other possible reasons could be shortages of personal protective equipment (PPE), but PPE supplies were monitored weekly, and supplies were adequately available. The other possibility would be contact with the patients while giving care. This was the case in 6.0% only, as the compliance with infection control practices was strictly monitored by ward in-charges. In addition, the infection prevention and control practitioners monitored the compliance during daily rounds, frequent audits, and training.
Working in a COVID-19 area was significantly associated with a higher risk of acquiring infection from a healthcare setting than from a community. A similar finding was also reported by Al Maskeri et al,7 where healthcare associated infections were also significantly higher among staff members working in COVID-19 areas (p < 0.001). This could be due to prolonged and repeated exposure to COVID-19 infected individuals.
During this study, the peak number of cases was noted from 5–11 July 2020 where most cases were thought to have been contracted from the community rather than hospital. The peak of COVID-19 infections in the general population was reported at about the same time, over 2000 cases, which then gradually declined.12
In our study, we found 18.9% of tested HCWs had no detectable IgG antibodies in their sera. Similarly, a large study from a Spanish hospital found 15% seronegativity among RT-PCR confirmed infected HCWs.4 Another study from the UK found that only 0.2% of the previously PCR-positive HCWs were seronegative.13 It could be possible that seronegative HCWs in our study had very low peak titers or had waning of their antibody levels that the analyzer could not detect despite the fact they were tested between 21 to 140 days of clinical presentation and positivity of RT-PCR. In addition, the N2 antibodies are less stable than antispike antibodies, and this might have contributed to seronegativity in some cases. Subsequent longitudinal cohort serological studies by Lumley and colleagues from the UK found waning anti-N2, but stable antispike antibodies.13
Antibody level was reported to peak at day 20–25 of symptoms.4 Furthermore, none of this cohort presented with re-infection (at least until the submission of this article), which could suggest that HCWs previously infected with COVID-19 might still have an element of protection, even in the absence of antibodies, possibly due to cell-mediated immunity.14 The duration of this protection could not be determined from this study, and eliciting that may require further follow-up with repeated serology testing.
Immunological response varies between patients following either infection or vaccination, including the level of formed antibodies and their sustainability.15,16 This interesting fact was evident in the statistically significant difference in the seropositivity rates to SARS-CoV-2 between Omani and non-Omani HCWs. We found non-Omanis had higher rates of seropositivity and the median level of IgG antibodies. The etiology behind this difference is uncertain. A similar response to vaccination will be worth studying and to understand contributing factors. Further, there was no significant difference between the age groups and genders in seropositivity, which is in line with findings by others.4 In contrast to the findings by Garcia-Basteiro et al,4 higher seropositivity was found in those working in COVID-19 areas in our study. Repeated exposure to the virus might explain this.
Garcia-Basteiro, et al4 found a significant correlation of some symptoms with seropositivity, including (in order): anosmia, ageusia, fever, and fatigue.4 Anosmia and ageusia were also found to have increased the odds of seroconversion compared to other symptoms in a New York City hospital study.3 In our cohort, only seven HCWs had anosmia, and two had ageusia, which might not have provided enough power to the study to show associated significance. However, out of the seven with anosmia, four were positive for IgG and one negative, while the other two did not come for testing. For ageusia, one was positive, and the other was not tested.
Our study has several limitations. First, it included only one center with a small number of subjects and a subgroup investigated for serological response. Nonetheless, it is the only study that provides an insight into serological testing to date in Oman. Second, serological testing was performed only once, while serial testing would have been optimal and provided a more comprehensive picture of the serological response to COVID-19. However, this data still provides a window of what initially occurs as a serological response to a novel virus in a pandemic situation. A larger multicenter study with follow-up of serial antibody measurements would provide a better understanding of the immunological response among this important group to set up policies and guidelines on quarantine and vaccination policies.
Conclusion
Nurses and other HCWs in COVID-19 areas are at the highest risk of contracting hospital-acquired infection. This, in turn, emphasizes the importance of adherence to infection control measures to prevent these infections. It also outlines high seropositivity among COVID-19 infected HCWs. These findings support the national guidelines that priority for vaccination should be given to HCWs in COVID-19 areas with no previous laboratory-confirmed infection.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
Acknowledgements
The authors wish to acknowledge the valuable contribution of nursing and laboratory departments at Al Nahdha Hospital in blood sample collection and analysis. In addition, we would like to thank Dr. Jawad al Lawati, senior consultant at the Ministry of Health, for his assistance in finalizing the manuscript.
references
- 1. Ducharme J. World health organization declares COVID-19 a Pandemic. Here’s what that means. 2021 [cited 2021 January 27]. Available from: https://time.com/5791661/who-coronavirus-pandemic-declaration/.
- 2. Lan FY, Wei CF, Hsu YT, Christiani DC, Kales SN. Work-related COVID-19 transmission in six Asian countries/areas: a follow-up study. PLoS One 2020 May;15(5):e0233588.
- 3. Venugopal U, Jilani N, Rabah S, Shariff MA, Jawed M, Mendez Batres A, et al. SARS-CoV-2 seroprevalence among health care workers in a New York City hospital: a cross-sectional analysis during the COVID-19 pandemic. Int J Infect Dis 2021 Jan;102:63-69.
- 4. Garcia-Basteiro AL, Moncunill G, Tortajada M, Vidal M, Guinovart C, Jiménez A, et al. Seroprevalence of antibodies against SARS-CoV-2 among health care workers in a large Spanish reference hospital. Nat Commun 2020 Jul;11(1):3500.
- 5. Feehan AK, Velasco C, Fort D, Burton JH, Price-Haywood EG, Katzmarzyk PT, et al. Racial and workplace disparities in Seroprevalence of SARS-CoV-2, Baton Rouge, Louisiana, USA. Emerg Infect Dis 2021 Nov;27(1):27.
- 6. Payne DC, Iblan I, Rha B, Alqasrawi S, Haddadin A, Al Nsour M, et al. Persistence of antibodies against Middle East respiratory syndrome coronavirus. Emerg Infect Dis 2016 Oct;22(10):1824-1826.
- 7. Al Maskari Z, Al Blushi A, Khamis F, Al Tai A, Al Salmi I, Al Harthi H, et al. Characteristics of healthcare workers infected with COVID-19: a cross-sectional observational study. Int J Infect Dis 2021 Jan;102:32-36.
- 8. Kluytmans-van den Bergh MF, Buiting AG, Pas SD, Bentvelsen RG, van den Bijllaardt W, van Oudheusden AJ, et al. Prevalence and clinical presentation of health care workers with symptoms of coronavirus disease 2019 in 2 Dutch hospitals during an early phase of the pandemic. JAMA Netw Open 2020 May;3(5):e209673.
- 9. CDC COVID-19 Response Team. Characteristics of health care personnel with COVID-19 - United States, February 12-April 9, 2020. MMWR Morb Mortal Wkly Rep 2020 Apr;69(15):477-481.
- 10. Khamis F, Al Rashidi B, Al-Zakwani I, Al Wahaibi AH, Al Awaidy ST. Epidemiology of COVID-19 infection in Oman: analysis of the first 1304 cases. Oman Med J 2020 Jun;35(3):e145.
- 11. Rappleye E. Gender ratio of nurses across 50 states. 2021 [cited 2021 January 27]. Available from: https://www.beckershospitalreview.com/hr/gender-ratio-of-nurses-across-50-states.html.
- 12. Directorate General of Diseases Surveillance & Control, Ministry of Health. Epidemiological situation of COVID-19 in the Sultanate till the end of July 2020. 2020 [cited 2021 January 27]. Available from: https://www.moh.gov.om/en/-/-19-2020.
- 13. Lumley SF, O’Donnell D, Stoesser NE, Matthews PC, Howarth A, Hatch SB, et al. Antibody status and incidence of SARS-CoV-2 infection in health care workers. N Engl J Med 2020;384(6):533-540.
- 14. Cox RJ, Brokstad KA. Not just antibodies: B cells and T cells mediate immunity to COVID-19. Nat Rev Immunol 2020 Oct;20(10):581-582.
- 15. Huang AT, Garcia-Carreras B, Hitchings MD, Yang B, Katzelnick LC, Rattigan SM, et al. A systematic review of antibody mediated immunity to coronaviruses: kinetics, correlates of protection, and association with severity. Nat Commun 2020 Sep;11(1):4704.
- 16. Xiang F, Wang X, He X, Peng Z, Yang B, Zhang J, et al. Antibody detection and dynamic characteristics in patients with coronavirus disease 2019. Clin Infect Dis 2020 Nov;71(8):1930-1934.