The number of cases affected by coronavirus disease 2019 (COVID-19) and related complications and deaths are dramatically increasing worldwide.1 Currently, the COVID-19 pandemic has affected 77 169 291 individuals and has caused 1 699 560 deaths globally.2 To date, there is no single effective therapeutic agent for COVID-19 infection. Standard supportive care, including oxygen supply and intensive care unit (ICU) support, is the main management modalities for critically ill patients. Several other investigational therapeutic options are currently being evaluated as potential therapies to be added to supportive care.3
Providing passive immunization in the form of convalescent plasma (CP) infusion that contains adequate neutralizing antibodies against severe acute respiratory syndrome coronavirus 2 (SARS-COV-2) is a potential therapeutic option that is currently being evaluated in various clinical trials.3–5 CP therapy has been used in the past to treat several other viral diseases. It has been effective in the treatment of SARS, Ebola virus (EBOV), Middle East Respiratory Syndrome (MERS), and H1N1 influenza.6–8 Similarly, evidence from earlier un-controlled case series from China on the use of CP in patients with COVID-19 infection has shown encouraging results favoring its use for severely ill patients. These studies have demonstrated clinical and laboratory improvements measured by reduction of oxygen requirements and mechanical ventilation (MV), improvement of the radiological findings, clearance of the virus, and normalization of laboratory parameters.4,5
Given the public health emergency of the COVID-19 pandemic, the Food and Drug Administration (FDA) permits the use of CP for COVID-19 patients through three pathways. First, as an investigational therapeutic option, via single patient emergency Investigations New Drug (eIND) applications, and through expanded use.9 Several studies are currently being conducted to evaluate the safety and efficacy of CP for the treatment of COVID-19. Recent publications have demonstrated high safety profile of CP therapy for patients as no major untoward events have been reported.10–13 Despite the methodological limitations of these studies, the data suggest some clinical benefits.
Nevertheless, the potential clinical benefit and risk of CP in COVID-19 remain uncertain due to the use of several other supportive interventions and the lack of wide-scale and well-designed randomized clinical trials. Therefore, the purpose of this study was to describe the initial clinical experience with CP transfusion administered to critically ill patients on MV with COVID-19 infection in Oman.
Methods
We conducted an open-label trial in Royal Hospital; a tertiary care hospital, in Muscat, Oman. The study was conducted from 17 April to 20 June 2020, and it compared two different treatment modalities of COVID-19 patients, CP with the standard of care versus standard of care alone. The standard care group was a historical control who were admitted at the same hospital from 12 March to 16 April 2020. Both groups received the standard of care for ICU patients that included hydroxychloroquine and lopinavir/ritonavir as per the National Guidelines (National Clinical Management Pathways for Hospitalized Patients with COVID-19, Ministry of Health, Oman, April 2020).14
The study was approved by the Royal Hospital Research and Ethics Committee (SRC#36/2020) and written informed consent was obtained from the patient or through their health proxy if intubated.
CP was collected from patients who had recovered from SARS-COV-2 and completed 14 days symptom-free. CP donors were selected based on the National Blood Donor Selection Criteria (NBDSC), which includes weight > 50 kg and age range between 18–65 years. Standard pre-donation assessment was conducted for each donor, and pre-screening tests were performed, including blood group, serological tests for transfusion-transmitted infections (TTI), and SARS-COV-2 immunglobulin G (SARS-COV-2 IgG) level.
The collection was performed using the apheresis procedure, and the volume collected was adjusted by gender, height, and weight and according to standard policy procedures. Each donor was tested again for the blood group and TTI by both nucleic acid amplification technique (NAT) and serology at the time of the donation. The plasma apheresis units were then processed in the laboratory and divided into two to three aliquots with a volume ranges from 200–250 mL and stored at -80 oC. CP units from all the blood groups were collected to meet the demands of patients. The units were stored at the blood service and issued to the hospital blood bank on request. No neutralizing antibody titer of the donors or patients was measured due to the global unavailability of the needed equipment and reagents during the period from April to June 2020. However, all eligible donors were tested for the SARS-CoV-2 IgG antibodies by the enzyme-linked immunosorbent assay (ELISA) method, which gives semi-quantitative IgG levels measurement. Only units that tested positive for the SARS-CoV-2 IgG were issued. In the cohort that received CP, six patients were excluded due to the unavailability of matched plasma.
The study included patients ≥ 18 years of age admitted with polymerase chain reaction (PCR) confirmed COVID-19 pneumonia with one of the following high-risk criteria:
- Critical respiratory condition or rapidly increasing oxygen requirement requiring MV.
- Severe pneumonia or ARDS with one of the following additional risk factors for complicated disease: age ≥ 60 years, immunodeficiency, hypertension, diabetes mellitus, coronary heart disease (CHD), chronic obstructive pulmonary disease (COPD), lymphocyte count < 0.8 × 109/L, lactate dehydrogenase (LDH) > 250 U/L, D-dimer > 1 µg/mL, and serum ferritin > 300 µg/L.
The study exclusion criteria were: patient’s rejection of plasma therapy, known IgA deficiency, hypersensitivity reaction to blood or blood products, history of severe transfusion reactions, unavailability of matching plasma, and illness lasting >14 days. The patients received 200 mL of CP at enrollment (day 0). A second dose was given 24–48 hours after the first dose in case the patient did not significantly improve and/or remained in critical respiratory condition.
Data gathered included demographics, baseline characteristics, risk factors, sequential organ failure assessment (SOFA) score, respiratory parameters (fraction of inspired oxygen (FiO2), positive end-expiratory pressure (PEEP), partial pressure of arterial oxygen (PaO2)/FiO2), pre-intervention on day 0, and post-intervention on days three, seven, and 14. In addition, data collected included laboratory parameters (absolute lymphocyte count (ALC), C-reactive protein (CRP), LDH, serum ferritin, D-dimer, interleukin-6 (IL-6), pH, and lactate) pre-intervention on day 0, and post-intervention on days three, seven, and 14.
The primary outcomes included extubation rates, discharges from the hospital, and mortality rates. Secondary outcomes included length of stay and improvements in respiratory and laboratory parameters.
- ARDS was defined as acute-onset hypoxemia (the ratio of PaO2:FiO2 of < 300) with > 50% bilateral pulmonary opacities on chest imaging within 24–48 hours that were not fully explained by congestive heart failure.15
- Pneumonia in adults was defined as evidence of lower respiratory tract infection, including difficulty in breathing, fast breathing > 20 breaths/min, crackles on examination, or new infiltrates on chest X-ray.
- Severe pneumonia in adults was defined as respiratory infection with fever and one of the following: respiratory rate of > 30 breaths/min, severe respiratory distress, and oxygen saturation (SpO2) of < 90% on room air.16
- Critical respiratory condition requiring high-flow nasal cannula, non-invasive ventilation (NIV), MV, or rapidly increasing oxygen requirement.
- Sepsis, defined as life-threatening organ dysfunction caused by a dysregulated host response to infection.17
- Septic shock in adults was defined as sepsis with persisting hypotension despite volume resuscitation, requiring vasopressors to maintain mean arterial pressure of ≥ 65 mmHg and serum lactate level of > 2 mmol/L.
- Multiple organ dysfunction syndrome (MODS) was defined as the progressive, potentially reversible dysfunction of two or more organ systems following acute, life-threatening disruption of systemic homeostasis.
Descriptive statistics was used to analyze the data. For categorical variables, frequencies and percentages were reported. Differences between groups were analyzed using Pearson’s chi-square tests (or Fisher’s exact tests for expected cells of < 5). For continuous variables, mean and standard deviation were used to summarize the data, while analyses were performed using Student’s t-test. Laboratory investigations and ventilatory parameters of the cohort stratified by CP over the hospital admission were analyzed using the repeated measures analysis of variance (ANOVA). The p-values for the differences over time were corrected using the Greenhouse-Geisser correction factor. Statistical studies were conducted using STATA version 16.1 (STATA Corporation, College Station, TX, USA).
Results
A total of 94 critically ill COVID-19 patients were enrolled in the study; 93.6% (n = 88) were on MV while 71.3% (n = 67) had ARDS. The overall mean age was 50.0±15.0 years, and 90.4% (n = 85) were males. A total of 77.7% (n = 73) of the patients had CP added to their medical management, in addition to the standard of care that was provided to all patients. The three most prominent symptoms observed were fever (86.2%; n = 81), shortness of breath (78.7%; n = 74), and cough (71.3%; n = 67). Other signs and symptoms as reported by the patients are shown in Figure 1.
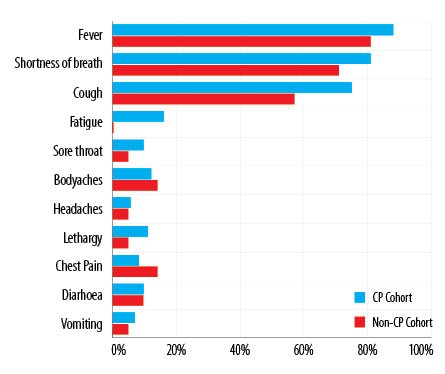
Figure 1: Signs and symptoms of the cohort stratified by convalescent plasma (CP).
Table 1: Demographic and clinical characteristics of the cohort with and without convalescent plasma.
|
Age, mean ± SD, years |
50.0 ± 15.0 |
53.0 ± 17.0 |
51.0 ± 15.0 |
0.644 |
|
Gender, male |
85 (90.4) |
19 (90.5) |
66 (90.4) |
0.993 |
|
Smoking, past/present |
4 (4.3) |
1 (4.8) |
3 (4.1) |
1.000 |
|
Hypertension |
35 (37.2) |
8 (38.1) |
27 (37.0) |
0.926 |
|
Diabetes mellitus |
34 (36.2) |
11 (52.4) |
23 (31.5) |
0.079 |
|
Chronic lung disease |
1 (1.1) |
1 (4.8) |
0 (0.0) |
0.223 |
|
Chronic heart disease |
7 (7.4) |
1 (4.8) |
6 (8.2) |
1.000 |
|
Chronic renal disease |
3 (3.2) |
1 (4.8) |
2 (2.7) |
0.536 |
|
Asthma |
2 (2.1) |
0 (0.0) |
2 (2.7) |
1.000 |
|
Pneumonia |
20 (21.3) |
2 (9.5) |
18 (24.7) |
0.135 |
|
Severe pneumonia |
7 (7.4) |
0 (0.0) |
7 (9.6) |
0.343 |
|
Sepsis/septic shock |
8 (8.5) |
0 (0.0) |
8 (11.0) |
0.192 |
|
X-ray findings |
|
|
|
|
|
Bilateral consolidations |
69 (73.3) |
13 (61.9) |
56 (76.7) |
0.261 |
|
Patchy reticular infiltrations |
22 (23.4) |
5 (23.8) |
17 (23.3) |
1.000 |
n (%) unless specified otherwise.
SD: standard deviation; SOFA: sequential organ failure assessment.
Table 2: Laboratory investigations and ventilatory parameters of the cohort stratified by convalescent plasma (CP) use.
|
WBC count, × 109/L |
8.7 vs. 10.2 |
11.8 vs. 10.1 |
12.2 vs. 11.9 |
15.9 vs. 13.4 |
< 0.001 |
0.693 |
|
ALC, × 109/L |
1.3 vs. 0.9 |
1.5 vs. 0.9 |
2.0 vs. 1.2 |
2.0 vs. 1.8 |
0.311 |
0.330 |
|
Hb, g/dL |
13.2 vs. 12.9 |
12.5 vs. 11.8 |
11.5 vs. 11.4 |
12.6 vs. 10.1 |
0.119 |
0.288 |
|
Platelets, × 109/L |
310 vs. 292 |
360 vs. 344 |
376 vs. 365 |
413 vs. 322 |
0.141 |
0.836 |
|
D-dimer, μg/mL |
7.1 vs. 9.1 |
3.4 vs. 8.4 |
3.9 vs. 7.4 |
6.0 vs. 8.5 |
0.970 |
0.915 |
|
CRP, mg/dL |
171 vs. 173 |
168 vs. 120 |
101 vs. 61 |
17 vs.19 |
0.005 |
0.365 |
|
Creatinine, μg/L |
99 vs. 95 |
122 vs. 122 |
107 vs. 140 |
53 vs. 122 |
0.592 |
0.930 |
|
ALT, U/L |
20 vs. 85 |
54 vs. 101 |
39 vs. 149 |
31 vs. 87 |
0.872 |
0.524 |
|
AST, U/L |
33 vs. 92 |
59 vs. 104 |
61 vs. 161 |
40 vs. 61 |
0.876 |
0.776 |
|
Total bilirubin, mmol/L |
16 vs. 13 |
11 vs. 22 |
9 vs. 15 |
11 vs. 9 |
< 0.001 |
0.839 |
|
Ferritin, μg/L |
1101 vs. 2744 |
443 vs. 1471 |
843 vs. 1362 |
561 vs. 942 |
0.979 |
0.780 |
|
LDH, U/L |
759 vs. 700 |
507 vs. 594 |
472 vs. 533 |
574 vs. 526 |
0.210 |
0.529 |
|
Corrected calcium, mmol/L |
2.1 vs. 2.1 |
17.9 vs. 4.2 |
2.3 vs. 2.2 |
2.2 vs. 2.2 |
0.066 |
0.482 |
|
IL-6, pg/mL |
166 vs. 427 |
577 vs. 840 |
2925 vs. 937 |
179 vs. 923 |
0.051 |
0.865 |
|
PO4, mg/dL |
1.6 vs. 1.4 |
1.7 vs. 1.2 |
1.7 vs. 1.5 |
1.2 vs. 1.6 |
0.785 |
0.321 |
|
PEEP, cm H20 |
13 vs. 12 |
13 vs. 11 |
13 vs. 10 |
10 vs. 10 |
0.007 |
0.304 |
|
FiO2, mmHg |
96 vs. 69 |
41 vs. 54 |
60 vs. 48 |
55 vs. 53 |
< 0.001 |
0.256 |
|
PaO2, mmHg |
86 vs. 80 |
98 vs. 88 |
67 vs. 82 |
77 vs. 88 |
0.662 |
0.793 |
|
pCO2, kPa |
46 vs. 44 |
50 vs. 46 |
60 vs. 46 |
- |
0.234 |
0.887 |
WBC: white blood cell; ALC: absolute lymphocyte count; Hb: hemoglobin; CRP: C-reactive protein; ALT: alanine transaminase; AST: aspartate aminotransferase; LDH: lactate dehydrogenase; IL–6: interleukin-6; PO4: phosphate; PEEP; positive end-expiratory pressure; FiO2: fraction of inspired oxygen; PaO2: partial pressure of arterial oxygen; pCO2: partial pressure of carbon dioxide; SpO2: oxygen saturation.
Hypertension (37.2%; n = 35), diabetes mellitus (36.2%; n = 34), and chronic heart disease (7.4%; n = 7) were the three most prevalent comorbidities. The overall median sequential organ failure assessment (SOFA) score was 5 (3–7). A total of 7.4% (n = 7) and 8.5% (n = 8) of the patients had severe pneumonia and sepsis/septic shock, respectively. X-ray findings indicated major bilateral consolidation opacities in 73.3% (n = 69) of the patients with 23.4% (n = 22) showing reticular interstitial patchy thickening in their X-rays. There were no significant differences among the demographic and clinical characteristics between the cohorts. Other details of the demographic and clinical characteristics are presented in Table 1.
There were no significant differences among the laboratory investigations and ventilatory parameters between the two cohorts as shown in Table 2. However, there were significant changes over time in the CP cohort with regards to the white blood cell (WBC) count (p < 0.001; increase), CRP (p = 0.005; decrease), total bilirubin (p < 0.001; decrease), PEEP (p = 0.007; decrease) and FiO2 (p < 0.001; decrease).
Those on CP were less likely to be prescribed azithromycin (1.4% vs. 57.1%; p < 0.001). Seventy percent (n = 66) of the patients in both groups received intravenous steroids. Patients in the CP group were less likely to be prescribed interferon beta 1B or peginterferon alpha-2a (6.8% vs. 71.4%; p < 0.001) compared to those that were not on CP. They also had a longer hospital stay length than those not on CP (12 vs. 8 days; p = 0.047). However, those on CP were more likely to be extubated (35.6% vs. 76.2%; p < 0.001) as well the higher composite endpoint of extubation/discharged home alive (64.4% vs. 23.8%; p = 0.001) when compared to those that did not receive CP. Furthermore, patients' CP also had the tendency for lower mortality when compared to COVID-19 patients that did not receive CP (19.2% vs. 28.6%; p = 0.354; study power = 11.0%). The list of other medications and clinical outcomes are outlined in Table 3.
Table 3: Medications and clinical outcomes of the cohort stratified by convalescent plasma use.
|
Antibiotic |
|
|
|
|
|
Ceftriaxone |
62 (66.0) |
14 (66.7) |
48 (65.8) |
0.938 |
|
Piperacillin |
69 (73.4) |
15 (71.4) |
54 (74.0) |
0.816 |
|
Meropenem |
30 (31.9) |
4 (19.0) |
26 (35.6) |
1.000 |
|
Azithromycin |
13 (13.8) |
12 (57.1) |
1 (1.4) |
< 0.001 |
|
Antiviral |
|
|
|
|
|
Lopinavir/Ritonavir |
79 (84.0) |
16 (76.2) |
63 (86.3) |
0.265 |
|
Antimalarial |
|
|
|
|
|
Hydroxychloroquine |
76 (80.9) |
18 (85.7) |
58 (79.5) |
0.754 |
|
Intravenous steroids |
66 (70.2) |
15 (71.4) |
71 (97.3) |
0.890 |
|
Interferons* |
20 (21.3) |
15 (71.4) |
5 (6.8) |
< 0.001 |
|
Outcomes |
|
|
|
|
|
Extubated |
42 (44.7) |
16 (76.2) |
26 (35.6) |
< 0.001 |
|
Remains hospitalized |
39 (41.5) |
9 (42.9) |
30 (41.1) |
0.885 |
|
Discharged home |
34 (36.2) |
5 (23.8) |
29 (39.7) |
0.181 |
|
Extubated/discharged home |
52 (55.3) |
5 (23.8) |
47 (64.4) |
0.001 |
n (%) unless specified otherwise.
*Included interferon beta 1B and peginterferon alpha-2a.
Discussion
We conducted an open-label trial analyzing the effectiveness of CP in COVID-19 infected patients that required MV and/or had ARDS. Both groups had similar demographics, baseline characteristics, and bilateral infiltrations on chest X-ray in accordance with the criteria for severe ARDS. In this study,18,19 many COVID-19 patients had ARDS caused by cytokine storm and host immune responses.CP was associated with higher rates of extubation as well as the composite endpoint of extubation/home discharges. The benefit of CP observed in these patients is partly hypothesized to be caused by neutralizing antibodies present in the donor CP that can provide high levels of passive antibodies titer until the host’s immune responses activates and clears both the viral infection from the blood circulation and pulmonary tissue as well as the infected cell.20–22
In the CP group, serial oxygenation parameters and laboratory investigations showed gradual improvement over time, including reduction in PaO2/FiO2 ratio, reduction in PEEP, increase in WBC count, and reduction in CRP and bilirubin. This was seen despite receiving less immunomodulating therapies such as interferon and azithromycin.23,24 Azithromycin was commonly used for bacterial respiratory infections and to treat or prevent co-infection with SARS-CoV-2 early in the pandemic. Azithromycin has shown in vitro antiviral activity against some RNA viruses, including Zika, rhinoviruses, and SARS-CoV-2.25,26 The use of azithromycin became less as studies showed a lack of efficacy and increased adverse events when combined with hydroxychloroquine.27 Similarly, interferon beta 1B or peginterferon alpha-2a was considered early into the pandemic for severe cases with evidence of cytokine storm.28
Although the improvement of inflammatory markers and oxygenation could be contributed to the adjunction of steroids, there has been a significant decrease in these markers in the CP group suggesting the additional potential role of the transfusion. The same observation was noted in previously published small case series studies.29–31 All investigated patients achieved serum SARS-CoV-2 RNA negativity after CP transfusion, accompanied by an increase of SpO2 and lymphocyte counts, and improved liver function and CRP. The results suggested that the inflammation and overreaction of the immune system were alleviated by the antibodies contained in CP.21
In the present study, 35.6% of patients receiving MV no longer required respiratory support after the CP transfusion. The beneficial effects could have been due to the transfusion of CP at the early stages of the disease, as neutralizing antibodies can wean quickly.20,21,32,33 In a recent multicenter study from Iran, the benefit from the CP transfusion was reported if CP was given early within three days of hospitalization and less than seven days of onset of the illness.10 In addition, all-cause mortality was reduced in the CP group compared to the standard care group (14.8% vs. 24.3%). However, similar to our study, this was not statistically significant, probably due to the low study power (11.0%).
Patients in our study that received CP were more likely to be extubated or discharged home than patients receiving the standard care only (23.8% vs. 64.4%, p = 0.001). Moreover, both groups equally received intravenous steroids (97.3% vs. 71.4%; p = 0.890). The case fatality rates (CFRs) in the CP group was 19.2%, which is comparable to the CFRs in four non-comparative studies using CP treatment.4,34–37 Similar to other reports, in the current study, no severe adverse effects, such as transfusion-related acute lung injury or antibody-dependent infection enhancement were observed or reported after CP transfusion.10,38–40
In this study, collection and transfusion of the plasma were done as previously reported, but there have been several technical limitations. Firstly, SARS-CoV-2 PCR was not repeated due to the limited availability of the testing early into the pandemic. Secondly, virus-specific neutralizing antibodies were not measured due to the unavailability of the tests. Virus-specific neutralizing antibodies are essential to accelerate the virus clearance and prevent further entry into target cells.41,42 However, CP units were given only if COVID-IgG antibodies were adequate after semi-quantitative measurement of the IgG levels. Thirdly, CP was not transfused on the same day of the collection, potentially affecting the antibody levels. Nevertheless, the beneficial effects of CP were observed in the clinical outcomes and laboratory responses. This is probably due to the proper selection process of donors who had recovered from SARS-COV-2 and the timing of their donation, which was at least four weeks from the onset of symptoms, to ensure adequate antibody titers. Recent studies have shown that SARS-CoV-2 viral neutralization activity correlates with the S protein receptor-binding domain (RBD), a key target for therapeutic antibodies that play a major part in tropism and virus entry into host cells and produces neutralizing antibodies and protective immunity.43,44 S-RBD-specific IgG are highest four weeks from the onset of symptoms; thus, we carefully selected the donors based both on this time period and on the IgG antibody levels that correlate well with neutralizing antibodies. Lastly, patients receiving CP were treated with other treatment modalities, including steroids. This could have potentially confounded the results, although patients in the CP group received less azithromycin and interferon. In fact, both groups received steroids equally, reflecting no major differences.
Conclusion
COVID-19 infected patients on MV and/or ARDS receiving CP tended to have better outcomes in terms of extubations and discharges. Based on our results, and in the absence of a specific treatment, CP therapy could have a clinical benefit in MV patients and could be a safe rescue option for severely ill COVID-19 patients. Large-scale randomized clinical studies are required to demonstrate the safety and efficacy of CP in COVID-19 patients.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
Acknowledgements
The authors would like to thank the participants for their corporation and providing informed consent and other necessary information. We also acknowledge the staff at the Royal Hospital who assisted us in the study.
references
- 1. Khamis F, Al Rashidi B, Al-Zakwani I, Al Wahaibi AH, Al Awaidy ST. Epidemiology of COVID-19 infection in Oman: analysis of the first 1304 cases. Oman Med J 2020 Jun;35(3):e145.
- 2. Wikipedia. COVID-19 pandemic by country and territory. [cited 2020 August 12]. Available from: https://en.wikipedia.org/wiki/COVID-19_pandemic_by_country_and_territory.
- 3. Abrams-Downey A, Saabiye J, Vidaurrazaga M. Investigational therapies for the treatment of COVID-19: updates from ongoing clinical trials. Eur Urol Focus 2020 Sep;6(5):1028-1031.
- 4. Shen C, Wang Z, Zhao F, Yang Y, Li J, Yuan J, et al. Treatment of 5 critically Ill patients with COVID-19 with convalescent plasma. JAMA 2020 Apr;323(16):1582-1589.
- 5. Duan K, Liu B, Li C, Zhang H, Yu T, Qu J, et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci U S A 2020 Apr;117(17):9490-9496.
- 6. Hui DS, Lee N. Adjunctive therapies and immunomodulating agents for severe influenza. Influenza Other Respir Viruses 2013 Nov;7(Suppl 3):52-59.
- 7. Hung IF, To KK, Lee CK, Lee KL, Chan K, Yan WW, et al. Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection. Clin Infect Dis 2011 Feb;52(4):447-456.
- 8. Luke TC, Kilbane EM, Jackson JL, Hoffman SL. Meta-analysis: convalescent blood products for Spanish influenza pneumonia: a future H5N1 treatment? Ann Intern Med 2006 Oct;145(8):599-609.
- 9. US FDA. Investigational COVID-19 convalescent plasma- emergency INDs. [cited 2020 August 12]. Available from: https://www.fda.gov/media/136470/download.
- 10. Abolghasemi H, Eshghi P, Cheraghali AM, Imani Fooladi AA, Bolouki Moghaddam F, Imanizadeh S, et al. Clinical efficacy of convalescent plasma for treatment of COVID-19 infections: results of a multicenter clinical study. Transfus Apher Sci 2020 Oct;59(5):102875.
- 11. Salazar E, Perez KK, Ashraf M, Chen J, Castillo B, Christensen PA, et al. Treatment of coronavirus disease 2019 (COVID-19) patients with convalescent plasma. Am J Pathol 2020 Aug;190(8):1680-1690.
- 12. Zhang B, Liu S, Tan T, Huang W, Dong Y, Chen L, et al. Treatment with convalescent plasma for critically Ill patients with severe acute respiratory syndrome coronavirus 2 infection. Chest 2020 Jul;158(1):e9-e13.
- 13. Joyner M, Wright RS, Fairweather D, Senefeld J, Bruno K, Klassen S, et al. Early safety indicators of COVID-19 convalescent plasma in 5,000 patients. medRxiv 2020 May.
- 14. National Clinical Management Pathways for Hospitalized Patients with COVID-19. MOH/UHAO/PRT/001/ Vers.01, Ministry of Health, Oman. [cited date]. Available from: https://www.moh.gov.om/documents/17733/4078547/MOH+Protocol+for+Management+of+Hospitalized+Adult+Patients+with+COVID-19+Infection/64946b17-5af5-e031-5672-28f986ebbf05.
- 15. Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, et al; ARDS Definition Task Force. Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012 Jun;307(23):2526-2533.
- 16. World Health Organization. Clinical management of COVID-19: interim guidance, 27 May 2020. World Health Organization.[cited date]. Available from: https://apps.who.int/iris/handle/10665/332196. License: CC BY-NC-SA 3.0 IGO.
- 17. Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016 Feb;315(8):801-810.
- 18. Channappanavar R, Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol 2017 Jul;39(5):529-539.
- 19. Rojas M, Rodríguez Y, Monsalve DM, Acosta-Ampudia Y, Camacho B, Gallo JE, et al. Convalescent plasma in Covid-19: Possible mechanisms of action. Autoimmun Rev 2020 Jul;19(7):102554.
- 20. Blanco-Melo D, Nilsson-Payant BE, Liu WC, Uhl S, Hoagland D, Møller R, et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell 2020 May;181(5):1036-1045.e9.
- 21. Halstead SB, Akkina R. COVID-19 and SARS coronavirus 2: antibodies for the immediate rescue and recovery phase. Front Immunol 2020 May;11:1196.
- 22. Jiang S, Hillyer C, Du L. Neutralizing antibodies against SARS-CoV-2 and other human coronaviruses. Trends Immunol 2020 May;41(5):355-359.
- 23. Ingraham NE, Lotfi-Emran S, Thielen BK, Techar K, Morris RS, Holtan SG, et al. Immunomodulation in COVID-19. Lancet Respir Med 2020 Jun;8(6):544-546.
- 24. Zimmermann P, Ziesenitz VC, Curtis N, Ritz N. The immunomodulatory effects of macrolides-A systematic review of the underlying mechanisms. Front Immunol 2018 Mar;9:302.
- 25. Bleyzac N, Goutelle S, Bourguignon L, Tod M. Azithromycin for COVID-19: more than just an antimicrobial? Clin Drug Investig 2020 Aug;40(8):683-686.
- 26. Fantini J, Chahinian H, Yahi N. Synergistic antiviral effect of hydroxychloroquine and azithromycin in combination against SARS-CoV-2: What molecular dynamics studies of virus-host interactions reveal. Int J Antimicrob Agents 2020;56(2):106020 .
- 27. Ramireddy A, Chugh H, Reinier K, Ebinger J, Park E, Thompson M, et al. Experience with hydroxychloroquine and azithromycin in the coronavirus disease 2019 pandemic: implications for QT interval monitoring. J Am Heart Assoc 2020 Jun;9(12):e017144.
- 28. Feuillet V, Canard B, Trautmann A. Combining Antivirals and Immunomodulators to Fight COVID-19. Trends Immunol 2020.
- 29. Chen L, Xiong J, Bao L, Shi Y. Convalescent plasma as a potential therapy for COVID-19. Lancet Infect Dis 2020 Apr;20(4):398-400.
- 30. Ahn JY, Sohn Y, Lee SH, Cho Y, Hyun JH, Baek YJ, et al. Use of convalescent plasma therapy in two COVID-19 patients with acute respiratory distress syndrome in Korea. J Korean Med Sci 2020 Apr;35(14):e149.
- 31. Ye M, Fu D, Ren Y, Wang F, Wang D, Zhang F, et al. Treatment with convalescent plasma for COVID-19 patients in Wuhan, China. J Med Virol 2020 Oct;92(10):1890-1901.
- 32. Casadevall A, Pirofski LA. The convalescent sera option for containing COVID-19. J Clin Invest 2020 Apr;130(4):1545-1548.
- 33. Abraham J. Passive antibody therapy in COVID-19. Nat Rev Immunol 2020 Jul;20(7):401-403.
- 34. Bloch EM, Shoham S, Casadevall A, Sachais BS, Shaz B, Winters JL, et al. Deployment of convalescent plasma for the prevention and treatment of COVID-19. J Clin Invest 2020 Jun;130(6):2757-2765.
- 35. Brown BL, McCullough J. Treatment for emerging viruses: Convalescent plasma and COVID-19. Transfus Apher Sci 2020 Jun;59(3):102790.
- 36. Zeng QL, Yu ZJ, Gou JJ, Li GM, Ma SH, Zhang GF, et al. Effect of convalescent plasma therapy on viral shedding and survival in COVID-19 patients. J Infect Dis 2020;222(1):38-43.
- 37. Tamburello A, Marando M. Immunoglobulins or convalescent plasma to tackle COVID-19: buying time to save lives - current situation and perspectives. Swiss Med Wkly 2020 Apr;150(1718):w20264.
- 38. Murphy M, Estcourt L, Grant-Casey J, Dzik S. International survey of trials of convalescent plasma to treat COVID-19 infection. Transfus Med Rev 2020 Jul;34(3):151-157.
- 39. Rajendran K, Krishnasamy N, Rangarajan J, Rathinam J, Natarajan M, Ramachandran A. Convalescent plasma transfusion for the treatment of COVID-19: Systematic review. J Med Virol 2020 Sep;92(9):1475-1483.
- 40. Joyner MJ, Bruno KA, Klassen SA, Kunze KL, Johnson PW, Lesser ER, et al. Safety update: COVID-19 convalescent plasma in 20,000 hospitalized patients. Mayo Clin Proc 2020 Sep;95(9):1888-1897.
- 41. Chowdhury MA, Hossain N, Kashem MA, Shahid MA, Alam A. Immune response in COVID-19: a review. J Infect Public Health 2020 Nov;13(11):1619-1629.
- 42. Seghatchian J, Lanza F. Convalescent plasma, an apheresis research project targeting and motivating the fully recovered COVID 19 patients: a rousing message of clinical benefit to both donors and recipients alike. Transfus Apher Sci 2020 Jun;59(3):102794.
- 43. Li L, Tong X, Chen H, He R, Lv Q, Yang R, et al. Characteristics and serological patterns of COVID-19 convalescent plasma donors: optimal donors and timing of donation. Transfusion 2020 Aug;60(8):1765-1772.
- 44. Salazar E, Kuchipudi SV, Christensen PA, Eagar TN, Yi X, Zhao P, et al. Relationship between anti-spike protein antibody titers and SARS-CoV-2 in vitro virus neutralization in convalescent plasma. bioRxiv 2020 Jun;2020.06.08.138990.