Rheumatoid arthritis (RA) is an autoimmune disease affecting any synovial joint but mainly involving the small joints of the hands and feet.1 Around half of patients develop an extra-articular manifestation of the disease, of which the most common are skin nodules, interstitial lung diseases, lymph node enlargement, and splenomegaly.2 In a cohort of 274 patients with extra-articular manifestations, cutaneous nodules were the commonest, seen in 17% of patients.3
Rheumatoid cutaneous nodules are granulomas with central fibrinoid necrosis that are surrounded by palisaded histiocytes. Similar granulomatous nodules can develop in visceral organs, mainly the lungs.4
A granuloma is defined as an aggregate of activated macrophages that acquire an epithelial-like morphological appearance, hence the name epithelioid histiocytes. Granulomas occur due to macrophages attempting to phagocytose microbes or products of tissue damage such as crystals or necrotic tissue.5 As such, they can arise due to several disease entities, with tuberculosis (TB) and sarcoidosis being the most common. Other causes include fungal infections, RA, gout, and cat-scratch disease among many others.6 Granulomas can be divided into immune and foreign body types. Foreign body granulomas result as a response to an inert material, such as surgical sutures, whereas immune granulomas are caused by certain agents that stimulate a cell-mediated immunologic reaction. In such a reaction, the activated T lymphocytes produce interferon-gamma, a potent activator of macrophages.7
Rheumatoid granulomas in the pancreas are rare, with only two reported cases in the literature.4,8
Here we report a case of a 55-year-old woman with a 15-year history of RA. The patient presented with epigastric pain and weight loss. Imaging showed a soft tissue mass in the head of the pancreas. The patient underwent a Whipple procedure for suspected cancer. Postoperative histological assessment revealed multiple granulomas consistent with rheumatoid nodules.
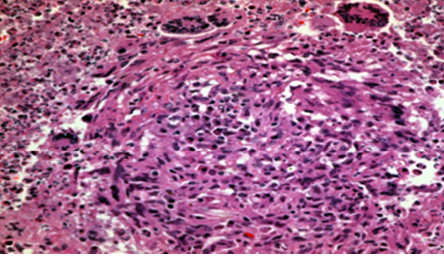
Figure 1: Pancreatic granuloma showing giant cells. Hematoxylin and eosin staining, magnification = 400 ×.
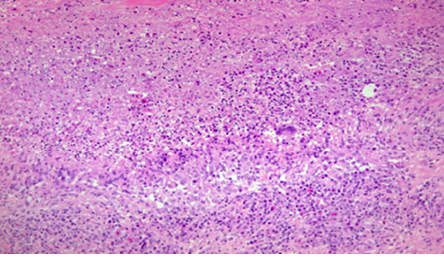
Figure 2: Pancreatic granuloma showing the peripheral palisade of histiocytic and neutrophilic debris. Hematoxylin and eosin staining, magnification = 200 ×.
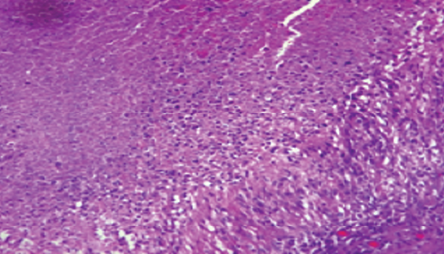
Figure 3: Pancreatic granuloma showing fibrinoid necrosis and histiocytic palisade. Hematoxylin and eosin staining, magnification = 200 ×.
Case report
A 55-year-old woman, was diagnosed with RA at the age of 40 years. She was initially treated with methotrexate for 18 months and since with betamethasone. She had no history of extra-articular manifestations. Rheumatoid factor at presentation was 70.6 IU/mL (normal level is < 14). The patient presented with epigastric pain of four months duration, radiating to the back, and associated with vomiting and loss of appetite. Also, the patient had unintentionally lost 10 kg of her weight over several months before presentation. There was no history of fever. Upon abdominal physical examination, there was no significant findings. Abdominal ultrasound imaging showed a well-defined lobulated soft tissue mass arising from the head of the pancreas that measured 3.6 × 4.2 × 5.3 cm. Intravenous contrast-enhanced computed tomography (CT) studies demonstrated a heterogeneous, lobulated, multi-cystic lesion with a necrotic center and peripheral enhancement. The lesion was located in the head of the pancreas and measured 3.8 × 3.7 × 4.8 cm with the impression of a serous cystic pancreatic tumor. Carbohydrate antigen 19-9 level was 407 U/mL (normal level in our lab is < 37), and carcinoembryonic antigen level was 2.6 ng/mL (normal level is < 5).
The Whipple procedure was done for suspected pancreatic cancer. The operation was uneventful. Specimens of the pancreas (6.5 × 6 × 4 cm), stomach (4 cm), duodenum (9 cm), and jejunum were sent for histopathological assessment. Macroscopically, the stomach, duodenum, and jejunum were unremarkable, and step sectioning of the pancreas revealed necrosis and cheesy material with no definite masses.
Microscopically, the stomach, duodenum, and jejunum were within normal histological limits. Seven benign lymph nodes had been identified with no granulomas. The pancreas was widely sampled. It showed a severe chronic inflammatory infiltrate with fibrosis and several epithelioid granulomas, some containing giant cells [Figure 1]. The majority of the granulomas exhibited large necrotic centers containing fibrin and neutrophils [Figure 2] and were surrounded by palisading histiocytes [Figure 3]. The features were those of granulomatous inflammation and there was no evidence of malignancy. There was no caseating necrosis, and Ziehl-Neelsen stain and polymerase chain reaction for suspected TB were negative. Periodic acid-Schiff stain for fungi was also negative. The histological features were in keeping with rheumatoid nodules. CT studies of the chest and abdomen showed no lesions.
There were no postoperative complications and the patient was continued on betamethasone as a treatment for RA. Three years after the operation, she is doing well without any complications.
Discussion
We report a rare case, the third of its kind, of rheumatoid granulomas arising within the pancreas. Preoperative assessment suggested a malignant neoplasm, whereas the postoperative histology ruled out malignancy and showed granulomatous inflammation.
Rheumatoid granulomas are described as necrotizing, palisading granulomas. They contain necrotic centers, but this is different from the central caseating necrosis seen in TB. Caseous necrosis in TB is composed of hyalinized, eosinophilic, acellular material, whereas the necrosis observed in necrotizing granulomas contains fibrin and cellular debris, hence the designation ‘dirty’ necrosis.9 The histopathological features in our case were typical of necrotizing palisading granulomas. These features are not specific to RA as they can be seen in infections, including fungal infections. Our patient did not have an infection on clinical assessment.
Around half of RA patients develop extra-articular manifestations, of which cutaneous rheumatoid granulomas are the commonest.2 Similar visceral granulomas are rarely described in the lungs, and there are only two published cases in the literature describing rheumatoid granulomas in the pancreas. One of the reported cases was a 64-year-old woman with an eight-year history of RA who presented with chronic abdominal pain and weight loss. Imaging studies revealed a 4.3 cm mass in the tail of the pancreas suggesting a malignant neoplasm. Histology revealed rheumatoid granulomas.8 The other case was a 49-year-old woman who presented with abdominal pain. Imaging studies showed multiple poorly circumscribed masses in the body and tail of the pancreas. Distal pancreatectomy was performed, and histology confirmed rheumatoid granulomas.4
Both cases share similar characteristics with our case, including the history of abdominal pain and weight loss, the preoperative suspicion of cancer, and the identical histological features. In the previous cases, the granulomas were located in the tail of the pancreas, whereas they were seen within the pancreatic head in our case.
Conclusion
Patients with long-standing RA can develop pancreatic granulomas, which might mimic cancer. Awareness among rheumatologists, gastrointestinal surgeons, and pathologists of this extra-articular manifestation is important to reach a correct diagnosis and not to confuse such granulomas with infectious or other disease processes.
Disclosure
The authors declared no conflicts of interest. Consent was obtained from the patient.
references
- 1. Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet 2010 Sep;376(9746):1094-1108.
- 2. Das S, Padhan P. An overview of the extraarticular involvement in rheumatoid arthritis and its management. J Pharmacol Pharmacother 2017 Jul-Sep;8(3):81-86.
- 3. Richman NC, Yazdany J, Graf J, Chernitskiy V, Imboden JB. Extraarticular manifestations of rheumatoid arthritis in a multiethnic cohort of predominantly Hispanic and Asian patients. Medicine (Baltimore) 2013 Mar;92(2):92-97.
- 4. Riedlinger WF, Lairmore TC, Balfe DM, Dehner LP. Tumefactive necrobiotic granulomas (nodulosis) of the pancreas in an adult with long-standing rheumatoid arthritis. Int J Surg Pathol 2005 Apr;13(2):207-210.
- 5. James DG. A clinicopathological classification of granulomatous disorders. Postgrad Med J 2000 Aug;76(898):457-465.
- 6. Ohshimo S, Guzman J, Costabel U, Bonella F. Differential diagnosis of granulomatous lung disease: clues and pitfalls: number 4 in the Series “Pathology for the clinician” Edited by Peter Dorfmuller and Alberto Cavazza. European respiratory review: an official journal of the European Respiratory Society 2017;26(145).
- 7. Timmermans WM, van Laar JA, van Hagen PM, van Zelm MC. Immunopathogenesis of granulomas in chronic autoinflammatory diseases. Clin Transl Immunology 2016 Dec;5(12):e118.
- 8. Nahm CB, Mittal A, Gill AJ, Samra J. Pancreatic rheumatoid nodule: case report and review of the literature. Pancreas 2017 May/Jun;46(5):e45-e47.
- 9. Aubry MC. Necrotizing granulomatous inflammation: what does it mean if your special stains are negative? Modern Pathology 2012;25(Suppl 1):S31-S38.