Dedifferentiated chondrosarcoma (DDC) is a rare malignancy that accounts for 11% of all chondrosarcoma cases and represents a distinct subtype.1 Most patients are over 50 years old and manifest with localized pain, pathologic fracture, and paresthesia. Morphologically, it comprises a low-grade chondroid tumor and a high-grade sarcoma with a sharp transition between them. The disease is aggressive with a dismal prognosis. Most cases arise de novo in axial bones such as the pelvis, femur, and humerus.1 Metastasis to the thyroid gland, which we will discuss in this report, is rare.
Case report
A previously healthy, 54-year-old man presented to Jordan University Hospital with right hip pain. Upon physical examination, the patient was limping and unable to bear his weight. Moreover, he notably had a palpable anterior neck mass. A neck ultrasound study was requested along with other radiologic workup for the right hip. The ultrasound showed a single, well-defined heterogeneous nodule, mainly hypoechoic, with a cystic component measuring 2.6 × 2.4 cm on the left thyroid lobe. Fine needle aspirate (FNA) biopsy from the thyroid mass showed numerous tight clusters of spindle cells [Figure 1]. The nuclei were intermediate in size and demonstrated a moderate degree of atypia and notable mitosis and apoptosis [Figure 1]. Immunohistochemical staining was performed on cell block. The spindle cells were positive for vimentin and negative for cytokeratin [Figure 2], thyroid transcription factor-1, and thyroglobulin. The provisional diagnosis was positive for malignant cells with features suggestive of sarcoma.
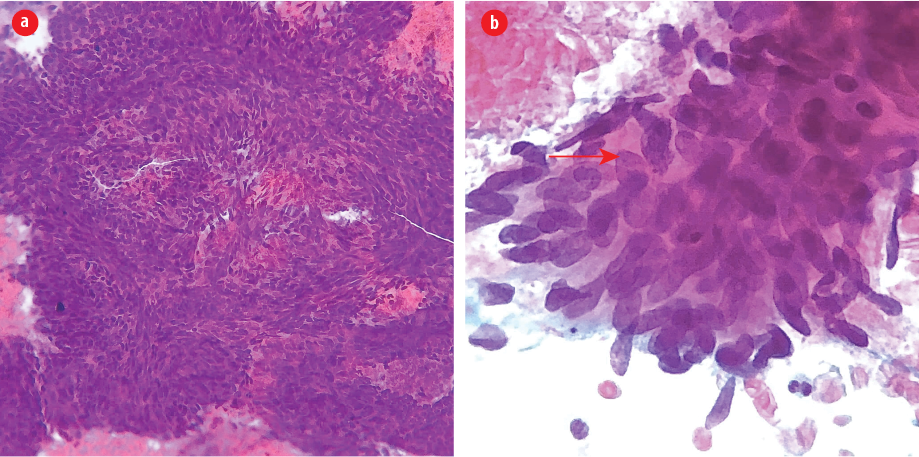
Figure 1: (a) Fine-needle aspirate from thyroid nodule revealed large aggregates of cohesive spindle cells in a hemorrhagic background (Pap stain, magnification = 200 ×). (b) Note the large size of tumor nuclei compared to surrounding cells and a neutrophil (below). Mitotic figures and irregular nuclear membrane are evident (arrow). Note also the thick overlapping of nuclei, indicating its complex architecture (Pap stain, magnification = 600 ×).
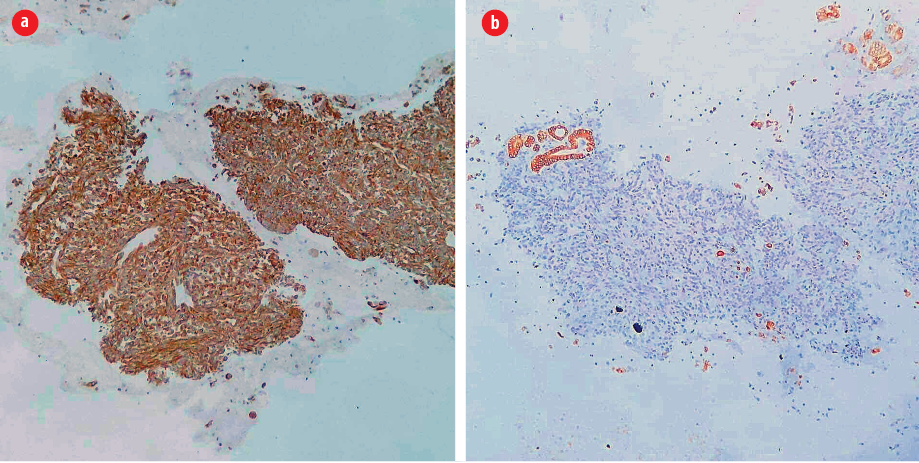
Figure 2: (a) The spindle cells are positive for vimentin (vimentin immunohistochemical stain, magnification = 100 ×) but (b) negative for cytokeratin, indicating their mesenchymal nature. Note the positivity in background follicular epithelial cells (cytokeratin immunohistochemical stain,
magnification = 100 ×).
Imaging studies of the hip showed a sizeable lytic mass in the right trochanteric region extending 15 cm in the femur shaft with a pathologic fracture. The patient underwent surgical fixation of the fracture, and a biopsy of the mass was sent for histopathology. Microscopic examination showed a biphasic tumor composed of fragments of a low-grade chondrosarcoma abutting areas of high-grade spindle cell sarcoma, characteristic of DDC. The morphologic and immunophenotypic features of the spindle cells were identical in both thyroid FNA and bone biopsy. Hence, the thyroid FNA was labeled as metastatic DDC. Later, a positron emission tomography scan showed hypermetabolic malignant processes involving both lungs, multiple visceral lymph nodes, duodenum, pancreas, liver, a peritoneal deposit, right femur, multiple bones, muscles, cutaneous foci, and left thyroid, which were consistent with widespread metastasis.
Discussion
Metastatic diseases in the thyroid gland are rare and account for < 0.2% of all thyroid cancers.2 Patients frequently present with a solitary palpable mass and are already known to have cancer. Yet, in a significant percentage of patients (20%–40%), the primary tumor is occult. Renal, breast, and lung carcinomas represent the most common origins.3 The low incidence of metastasis to the thyroid despite its high vascularity was attributed to its special microenvironment of high-speed arterial blood flow and the high oxygen and iodine content, which impede tumor anchoring and growth.4 However, postmortem examination of patients who died of cancer showed histologic evidence of thyroid metastasis in up to 24%, suggesting a frequent subclinical metastasis. Thyroidectomy would also help diagnose the primary tumor and improve morbidity in isolated metastatic cases, but not in widespread metastasis.3
In clinical practice, it is rare to encounter a spindle cell neoplasm in thyroid FNA specimens. Although the hallmark of mesenchymal tumors, both benign and malignant, is being spindle shaped, this can take place in some epithelial tumors too. Medullary thyroid carcinoma, anaplastic carcinoma, and spindle epithelial tumor with thymus-like differentiation tumor can have a spindle morphology, yet, these tumors are epithelial in origin and thus are positive for cytokeratin immunohistochemical stain.5 In contrast, mesenchymal tumors are generally negative for cytokeratin and positive for vimentin and other lineage-specific markers.
Sarcoma of the thyroid gland can be primary or metastasic. Primary thyroid sarcomas are rare, usually reported only as case reports such as chondrosarcoma, Kaposi sarcoma, leiomyosarcoma, synovial sarcoma, and undifferentiated sarcoma.5 Similarly, metastatic sarcomas represent 2% of metastatic cancers in the thyroid gland, indicating its exceptional rarity.3 In an article published in 2019, the authors reviewed the literature for metastatic chondrosarcoma in the thyroid gland and found only six cases, including theirs.6 DDC was present in one case only, while the remaining cases were conventional and mesenchymal types. In the case of DDC, the patient developed metastasis a year after the diagnosis and treatment of the primary tumor.7 In contrast, our patient had thyroid metastasis at the initial time of diagnosis of femur DDC.
DDC is a rare but highly malignant cancer with a very poor prognosis. Most cases arise de novo, and the most common sites are pelvis, femur, and humerus. Most patients have metastasis at the time of diagnosis and die within two years.1 Our patient had a widespread visceral and bony metastasis, with histologic evidence from the thyroid gland. Due to the rarity of thyroid metastasis, the pathologic diagnosis was established after correlation with the findings from the bone biopsy. The morphology and the immunophenotype were identical in the two specimens.
Conclusion
The thyroid gland remains an infrequent and unusual site for metastatic DDC, especially as a presenting sign. Therefore, a high index of suspicion should be raised when encountering a tumor with spindle cell morphology. The key factors in reaching the correct diagnosis are good morphologic skills, immunohistochemical stains, and correlation with clinical status. By reporting our cases and reviewing the literature, we hope to increase awareness among physicians of the possibility of DDC metastasis to the thyroid gland.
Disclosure
The authors declared no conflicts of interest. The patient gave consent to publish this case report.
references
- 1. Liu C, Xi Y, Li M, Jiao Q, Zhang H, Yang Q, et al. Dedifferentiated chondrosarcoma: radiological features, prognostic factors and survival statistics in 23 patients. PLoS One 2017 Mar;12(3):e0173665.
- 2. Straccia P, Mosseri C, Brunelli C, Rossi ED, Lombardi CP, Pontecorvi A, et al. Diagnosis and treatment of metastases to the thyroid gland: a meta-analysis. Endocr Pathol 2017 Jun;28(2):112-120.
- 3. Nixon IJ, Coca-Pelaz A, Kaleva AI, Triantafyllou A, Angelos P, Owen RP, et al. Metastasis to the thyroid gland: a critical review. Ann Surg Oncol 2017 Jun;24(6):1533-1539.
- 4. Willis RA. Metastatic tumours in the thyreoid gland. Am J Pathol 1931 May;7(3):187-208.
- 5. Lloyd RV, Osamura RY, Kloppel G, Rosai J, editors. WHO classification of tumours of endocrine organs. 4th ed. Lyon: IARC; 2017.
- 6. Wu ZH, Dai JY, Shi JN, Fang MY, Cao J. Thyroid metastasis from chondrosarcoma. Medicine (Baltimore) 2019 Nov;98(47):e18043.
- 7. Darouassi Y, Touati MM, Chihani M, Nadour K, Boussouga M, Ammar H, et al. Chondrosarcoma metastasis in the thyroid gland: a case report. J Med Case Rep 2014 May;8(1):157.