Chondrosarcoma is a malignant tumor of cartilage origin, and its incidence rate is second among primary malignant bone tumors.1 It can be divided into different histological types according to histological characteristics and pathological sites. Besides, the tumors have considerable variation in outcome depending on size, histologic grade, Musculoskeletal Tumor Society (MSTS) stage, and tumor type. The incidence rate grading from a higher rate to a lower rate is as follows: the common central type, conventional, dedifferentiated type, mesenchymal, myxoid type, and clear cell type.1,2 The tumor grade and the anatomic site are important because both influence the type of treatment and outcome. Chondrosarcoma responds poorly to traditional chemotherapy and radiotherapy. The use of conventional adjuvant therapy for a higher-lower grade or advanced chondrosarcoma is still controversial.3–6 Surgical treatment is the only known treatment with definite results at present. The pelvis is the second most common site of chondrosarcoma.7,8 Due to the complicated and special anatomical structure, it is difficult for resection and reconstruction of the site; the intraoperative bleeding is high, and the incidence of surgical complications is high, so clinical treatment is always challenging.9 In this study, we retrospectively analyzed the medical records of 24 patients with pelvic chondrosarcoma, and summarized the clinical statistics evidence of pelvic chondrosarcoma and analyzed its prognostic factors.
Methods
According to the hospital medical record database, a total of 78 cases of chondrosarcoma were confirmed by pathological examination and admitted to the General Hospital between 2008 and 2017. Five cases were not included in the study due to lack of data. Out of the 73 patients, 24 had pelvic chondrosarcomas; 14 males and 10 females with a ratio of 1.4:1. The patients were aged from 22 to 69 years, with a median age of 43.5 years. Pelvic tumors were partitioned according to the Enneking system,10 with five cases grade I, 14 cases grade II, and five cases grade III. All patients accepted puncture or incision biopsy. There was one case of histological grading of chondrosarcoma grade I, 15 cases of grade II, and eight cases of grade III. The histological type was normal central type in 17 cases, dedifferentiated type in three cases, secondary type in two cases, and one case of myxoid and mesenchymal types each. To better understand all subtypes of chondrosarcoma, the authors collected all data, and these are summarized in Table 1.
All 24 cases of pelvic chondrosarcoma underwent surgical treatment, including 15 cases of resection and reconstruction in the first operation, eight cases of resection and no reconstruction, and one case of hip amputation (the tumor was huge and invaded major blood vessels and important nerves, so limb salvage operation was not possible). Sixteen patients underwent two or more operations due to local recurrence or postoperative dysfunction, of which 12 ended up with amputations. A total of five patients were treated with chemotherapy, including one preoperative chemotherapy and four postoperative chemotherapy. The chemotherapy regimen includes ifosfamide + doxorubicin + azolidazole, ifosfamide + doxorubicin, or methotrexate + epirubicin. Three patients were treated with postoperative radiotherapy.
Patients were followed up in the clinic once every three months to reexamine the surgical site’s X-ray, and chest radiographs repeated within two years of the surgery. Computed tomography (CT) or magnetic resonance imaging (MRI) was performed if required. If there were no signs of progress, the follow-up was changed to once every six months after two years and annually after five years. Follow-up records included local recurrence and distant metastasis. If the patient died, the time of death was confirmed by telephone follow-up. The duration of follow-up was defined as from the time of diagnosis until the patient died or was counted.
We used SPSS Statistics (IBM Corp. Released 2011. IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp.) for data collection and statistical analysis. Pearson’s chi-square test was used to test the correlation between the two categorical variables, and Fisher’s exact probability method was used if the frequency was < 5. Kaplan-Meier method was used to analyze survival and estimate survival rate. COX regression model was used for univariate analysis of survival time. When these variables are incorporated into the COX regression model for multi-factor analysis, the independent variables are screened by the advanced method based on maximum likelihood estimation. A p-value < 0.050 indicated that the difference was statistically significant.
Results
Chondrosarcomas of the pelvis were rarely classified as grade I, with only one in 24 (4.2%) compared to 26.5% in other chondrosarcomas. The local recurrence rate of pelvic chondrosarcoma was significantly higher than the other sites (83.3%; 20 cases vs. 34.7%; 17 cases). During treatment, the percentage of lesions in the pelvis that eventually underwent amputation was significantly higher than in non-pelvic areas (50.0% in 12 cases). Three patients (12.5%) with pelvic chondrosarcoma received postoperative radiotherapy, but no patients with tumors of other sites received radiotherapy. A total of 73 patients with chondrosarcoma were included in this study with a male to female ratio of 1.03:1, showing no significant gender bias. There was no statistically significant difference in the gender, age, chemotherapy, or distant metastasis between pelvic chondrosarcoma patients and non-pelvic chondrosarcoma patients [Table 2 and Figure 1].
Table 1: Overview of clinical characteristics and therapeutic options in all subtypes of bone chondrosarcoma.
|
All
chondrosarcoma, % |
~75 |
~10 |
~10 |
< 2 |
< 2 |
|
Precursor lesion |
Enchondroma (up
to 40%) |
Osteochondroma (100%) |
Conventional
chondrosarcoma |
None |
None |
|
Occurrence
Within syndrome |
Enchondromatosis
(Ollier disease) |
Multiple osteochondromas (MOs) |
Rarely in MOs or
enchondromatosis |
None |
None |
|
Age, years |
> 50 |
Younger than central
chondrosarcoma |
59 (median) |
Any age (peak in
second and third
decade) |
Any age (peak in
third to fifth
decade) |
|
Preferential
location |
Pelvis, proximal
femur, proximal
humerus, distal
femur, ribs |
Pelvis, shoulder girdle
bones |
Femur and pelvis |
65%–86% skeleton
(jawbones, ribs,
ilium, vertebrae)
14%–43%
Extra-osseous
(meninges) |
Epiphysis of
humeral or
femoral head |
|
Histological
grading |
Grade I–III |
High grade |
High grade |
Low grade |
- |
|
Survival |
Grade I: 83%;
Grade II: 64%;
Grade III: 29% at
10 years |
24% at 5 years |
28% at 10 years |
89% at 10 years |
- |
|
Sensitivity to
chemotherapy |
None |
None |
Uncertain |
Possibly, if high
percentage round cells |
None |
|
Sensitivity to
radiotherapy |
Low |
Low |
Low |
- |
Low |
BCL2: B-cell lymphoma 2 protein; COX-2: cyclooxygenase 2; ER: estrogen receptor; HDAC: histone deacetylase; IHH: Indian hedgehog; MMP: matrix metalloproteinase; PDGFR-a: platelet-derived growth factor receptor a; PTHLH: parathyroid hormone-like hormone; c-PKC-a: protein kinase C-alpha.
Table 2: Different characteristics between pelvic and non-pelvic chondrosarcomas.
|
Gender |
|
|
0.837 |
0.360 |
|
Female |
10 (41.7) |
26 (53.1) |
|
|
|
Male |
14 (58.3) |
23 (46.9) |
|
|
|
Age, years |
|
|
0.890 |
0.345 |
|
< 50 |
16 (66.7) |
27 (55.1) |
|
|
|
> 50 |
8 (33.3) |
22 (44.9) |
|
|
|
Histological grade |
|
|
5.307 |
0.070 |
|
I |
1 (4.2) |
13 (26.5) |
|
|
|
II |
15 (62.5) |
22 (44.9) |
|
|
|
III |
8 (33.3) |
14 (28.6) |
|
|
|
Amputation |
|
|
6.700 |
0.010 |
|
Yes |
12 (50.0) |
10 (20.4) |
|
|
|
No |
12 (50.0) |
39 (79.6) |
|
|
|
Chemotherapy |
|
|
- |
0.720* |
|
Yes |
4(16.7) |
6(12.2) |
|
|
|
No |
20 (83.3) |
43 (87.8) |
|
|
|
Radiotherapy |
|
|
- |
0.033* |
|
Yes |
3 (12.5) |
0 (0.0) |
|
|
|
No |
21 (87.5) |
49 (100) |
|
|
|
Local recurrence |
|
|
- |
< 0.001* |
|
Yes |
20 (83.3) |
17 (34.7) |
|
|
|
No |
4(16.7) |
32 (65.3) |
|
|
|
Metastasis |
|
|
0.991 |
0.319 |
|
Yes |
8 (33.3) |
11 (22.4) |
|
|
*Fisher’s exact test.
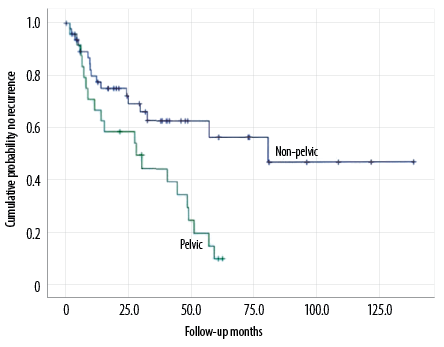
Figure 1: Local recurrence-free survival of pelvic and non-pelvic chondrosarcomas.
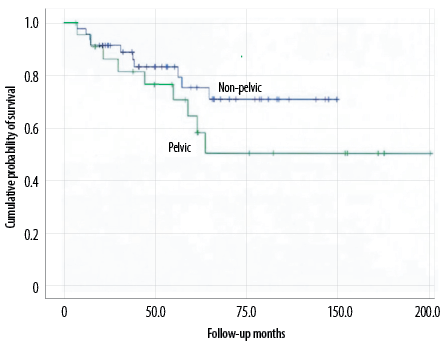
Figure 2: Overall survival of pelvic and non-pelvic chondrosarcomas.
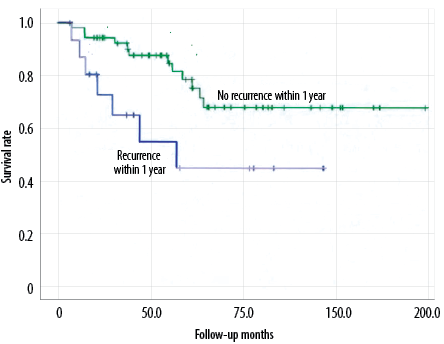
Figure 3: Effect of local recurrence within one year on overall survival of patients with pelvic chondrosarcomas.
Table 3: Univariate prognostic factors analysis of pelvic chondrosarcomas.
|
Gender |
|
|
0.780 |
|
Female |
10 |
0.360–3.899 |
|
|
Male |
14 |
|
|
|
Age, years |
|
|
0.130 |
|
< 50 |
16 |
0.118–1.317 |
|
|
> 50 |
8 |
|
|
|
Enneking stage |
|
|
0.379 |
|
I |
5 |
0.353–10.694 |
|
|
II |
14 |
0.148– 3.972 |
|
|
III |
5 |
|
|
|
Maximum diameter of tumor, cm |
0.280 |
|
< 8 |
10 |
0.586–6.327 |
|
|
> 8 |
14 |
|
|
|
Pathological grade |
0.759 |
|
I |
1 |
> 0.000 |
|
|
II |
15 |
0.194–2.093 |
|
|
III |
8 |
|
|
|
Amputation |
|
|
0.963 |
|
Yes |
12 |
0.287–3.288 |
|
|
No |
12 |
|
|
|
Chemotherapy |
|
|
0.297 |
|
Yes |
5 |
0.562–6.594 |
|
|
No |
19 |
|
|
|
Radiotherapy |
|
|
0.764 |
|
Yes |
3 |
0.271–5.920 |
|
|
No |
21 |
|
|
|
Local recurrence |
0.303 |
|
Yes |
20 |
0.048–17.239 |
|
|
No |
4 |
|
|
|
Time between surgery and recurrence, years |
0.001 |
|
< 1 |
8 |
3.197–90.745 |
|
CI: confidence interval.
Diagnostic methods for local recurrence or distant metastasis include CT, MRI, isotope bone scan, positron emission tomography (PET)-CT, and biopsy. In this study, five patients had Enneking grade I, two underwent reconstruction with allograft bone graft, and all recovered well. In cases of grade II hip joint tumor that requires surgery, they generally need to rebuild with restoring joint function. In this study, nine of the 14 patients were constructed with artificial weight (saddle prosthesis or artificial acetabular cup). Three of these patients had a deep infection, and two had dislocation; there were no complications in one case of artificial bone grafting and one case of pelvic amputation. Only three cases were cleared without reconstruction, one of which was complicated with deep postoperative infection. Among those patients with pelvic chondrosarcomas surgically removed, one had local recurrence. Recurrence interval refers to the time from surgery to local recurrence (if any), ranging from 1.7 to 59.4 months, with a median time of 21.7 months. The survival of patients with pelvic chondrosarcoma without local recurrence was shorter than patients with other tumors (p = 0.005). The time interval between surgery and distant metastasis was 5.2–66.0 months, and the median time was 32.4 months. The distant metastases included five cases of lung, one case of the abdominal cavity, one case of retroperitoneum, and one case of multiple metastases of lung and abdominal cavity. In comparison, patients with high differentiation in other parts accounted for 26.5%, indicating that chondrosarcoma of the pelvis was more malignant. In this study, pelvic chondrosarcoma was the second most common disease site after the femur accounting for 32.9%, which is slightly higher than similar studies.7,11
The median follow-up time of 73 patients with chondrosarcoma was 60.1 months, with 21 deaths, 50 cases surviving, and four cases missing in the follow-up. The overall survival rates at three, five, and 10 years were 86.9±4.1%, 79.5±5.2%, and 64.7±6.9%, respectively. The median follow-up time of 24 patients with pelvic chondrosarcoma was 67.4 months; 13 patients died, 11 survived, and two were lost to follow-up. The three, five, and 10-year survival rates were 82.2±8.1%, 77.3±8.9%, and 52.4±12.1%, respectively.
The total survival time of pelvic and non-pelvic chondrosarcoma patients was not significantly different (p = 0.216) [Figure 2]. The univariate analysis shows the overall survival time of patients with pelvic chondrosarcoma, including variables such as gender, age at diagnosis at < 50 years, tumor’s Enneking grade, maximum diameter < 8 cm, histological grade, final amputation, chemotherapy and radiotherapy treatment, recurrence, and whether the time from surgery to recurrence was < 1 year. The results showed a significantly shorter survival time for patients with local recurrence within one year (p = 0.001) [Figure 3]. Further multivariate analysis indicated that local recurrence was the only independent prognostic factor within one year. Patients’ gender, age, tumor site, size, histological grade, eventual amputation, chemotherapy or radiotherapy, and recurrence were unrelated to overall survival [Table 3].
Discussion
The pelvic anatomical structure is complicated. Pelvic tumor surgery is difficult with high risks and many postoperative complications. This study analyzes and summarizes the clinical statistics and prognostic factors of pelvic and non-pelvic chondrosarcoma to provide a reference for the clinical diagnosis, disease treatment, and improve the patient’s life quality. The age range was 22 to 69 years, with a median age of 43.5 years, showing demographic characteristics similar to previous studies.7,11,12 Pelvic tumors were partitioned according to Enneking,10 and the largest proportion of tumors were stage II (58.3%) because they involve the hip joint and increases the difficulty of surgery. The size of the tumor varied depending on whether it was located in the pelvis. The mean maximum diameter of the tumor in the pelvis was 10.5 cm, while that in other parts was 7.1 cm. There were also significant differences in tumor grades according to different sites. The histological manifestations of chondrosarcoma of the pelvis were few (4.2%), and most were of medium and low differentiation.
Pelvic chondrosarcoma is not easy to diagnose and treat early. The main reason is that there is ample space in the pelvis for tumor growth, resulting in obvious swelling, compression, pain, and other symptoms later. Chondrosarcoma should be highly suspected in cases of pelvic tumors, where a puncture or open biopsy indicates the origin of the tumor cartilage. If it is confirmed as a benign tumor, it should be followed up closely postoperatively and alert to secondary malignant changes. In this study, two cases (8.3%) were secondary chondrosarcomas of benign tumors. Surgical procedures for pelvic chondrosarcoma may be selected according to the Enneking subdivision. Attention should be paid to the operation because the sciatic nerve out of the outside of the pelvis often and has iliac vessels branch of the superior gluteal blood vessels through.The surgeon must not avoid the first ligation of the blood vessels to prevent uncontrolled bleeding.
Adequate soft tissue coverage is the key to the success of the operation. Compared with traditional customized prostheses, 3D printing can achieve a satisfactory reconstruction effect. This kind of prosthesis has been routinely used in our hospital in recent years because of its good angle matching in the acetabular cup and the difficulty of femoral head dislocation. If the tumor is grade III and does not involve the hip joint, only sitting and pubic resection do not affect the hip joint function. It can only be filled with a bone graft or rebuilt. Since the sciatic tubercle is closer to the sciatic nerve, the sciatic nerve should be fully exposed and protected during surgery to avoid injury. In this study, two of the five patients underwent artificial hip reconstruction due to the involvement of the hip joint, of which one had deep postoperative infections, two had resection only, and one had no significant complications after amputation. In cases of metatarsal tumors, if total metatarsal resection is performed, reconstruction is required. Generally, a screw rod system is used to connect the spine and the metatarsal bone to maintain the stability of the pelvic ring.13 This study did not include this type of case.
Non-surgical treatments for chondrosarcomas are limited, and the efficacy of chemotherapy for chondrosarcoma remains controversial.3–5 However, Kawaguchi et al,6 showed that for dedifferentiated chondrosarcomas, surgical resection combined with ifosfamide chemotherapy resulted in significantly longer disease-free survival than patients treated with surgery alone. Italiano et al,14 studied the response of advanced chondrosarcomas to chemotherapy (unretractable or with distant metastasis), showing that multi-drug combination chemotherapy can significantly delay the onset of disease progression, especially in patients with mesenchymal and dedifferentiated chondrosarcomas. Some scholars have also explored targeted therapy for chondrosarcoma, focusing more on chemokine receptor 4. This receptor can increase vascular endothelial growth factor expression, induce angiogenesis, and improve cell invasiveness.15 Sun et al,16 have successfully inhibited tumor growth and lung metastasis in a mouse chondrosarcoma transplantation model with its specific blocking drug AMD3100. Therefore, for mesenchymal and dedifferentiated chondrosarcomas, adjuvant chemotherapy can be used to control the progression of the disease if the tumor is characterized by rapid growth, blurred boundary, multiple postoperative recurrences, and other features indicating a high degree of malignancy. Further research is needed to find more sensitive drugs to standardize adjuvant therapy. It is generally believed that chondrosarcoma is not sensitive to radiotherapy, and possible reasons include the deletion of tumor suppressor gene P16 in chondrosarcoma cells and increased expression of anti-apoptotic proteins such as Bcl-2, Bcl-xL, and XIAP.17 Previous clinical studies also mostly confirmed that radiotherapy could not improve the prognosis of chondrosarcoma.18,19 However, a retrospective study of 31 cases of pelvic chondrosarcoma showed that carbon ion radiotherapy had no significant effect on the survival time of patients, and the limb function of the patients was significantly better than the surgery group.20
A clinical trial also showed that carbon-ion radiotherapy for middle and low-level cranial base chondrosarcoma could achieve better local control with fewer adverse reactions.21
Carbocation radiotherapy is a trial-and-true treatment option for pelvic chondrosarcoma patients who are difficult to treat surgically and not sensitive to chemotherapy. In this study, three patients with pelvic chondrosarcoma were treated with radiotherapy, two of whom were treated with radiotherapy after amputation and had died at the time of follow-up. In this study, pelvic chondrosarcoma was more likely to recur with a local recurrence rate of 83.3% after surgical resection. Combined with the existing studies, the local recurrence rate after resection varies greatly with different sites and operation methods. Roos et al,22 reported a local recurrence rate of 17.1% within five years of primary rib chondrosarcoma resection. Al-Refaie et al,23 reported a local recurrence rate of 4.4% after chest wall chondrosarcoma. Yin et al,18 reported the local recurrence rate of chondrosarcoma after surgery was 42.9%. Ogose et al,24 reported that the postoperative recurrence rate of hand and foot chondrosarcoma was 40.4%, and the recurrence rate was closely related to the surgical procedure. Tumor recurrence rates after amputation, lesion resection, and curettage decreased in turn, which suggests that sufficient resection range can reduce the local recurrence rate.
We found that recurrence did not affect the overall survival time of patients, but the prognosis of patients with earlier recurrence (< 1 year) was poor, which was consistent with the existing research results19 and may be a manifestation of the high malignant degree of the tumor. In this study, the proportion of pelvic chondrosarcoma with metastasis was 33.3%, and the sites were lung, abdominal cavity, and retroperitoneal. Previous studies have also shown that chondrosarcomas are most likely to metastasize to the lungs.18,25,26 Compared with other chondrosarcomas, the proportion of spinal metastasis was 24.5%,18 17.1%,24 and 11.8%.22 The probability of pelvic metastasis is relatively high, which is consistent with previous research results suggesting that the overall malignant degree of pelvic chondrosarcoma is higher than other sites.8
Our study has some limitations and may also have susceptible to bias associated with certain patients being reported over others, so further research should be conducted.
Conclusion
After surgical resection of pelvic chondrosarcoma, the local recurrence rate was significantly higher than other chondrosarcomas, and the final amputation rate was also significantly higher. Local recurrence within one year of surgery is an independent prognostic factor for pelvic chondrosarcoma. Some findings of this study are supported in previously published literature. Analysis of the difference between surgical techniques, clinical statistics, prognostic factors, and patient outcomes has been limited because of the rarity of these lesions and few institutions having enough patients to study about it.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
references
- 1. Giuffrida AY, Burgueno JE, Koniaris LG, Gutierrez JC, Duncan R, Scully SP. Chondrosarcoma in the United States (1973 to 2003): an analysis of 2890 cases from the SEER database. J Bone Joint Surg Am 2009 May;91(5):1063-1072.
- 2. Gelderblom H, Hogendoorn PC, Dijkstra SD, van Rijswijk CS, Krol AD, Taminiau AH, et al. The clinical approach towards chondrosarcoma. Oncologist 2008 Mar;13(3):320-329.
- 3. Staals EL, Bacchini P, Bertoni F. Dedifferentiated central chondrosarcoma. Cancer 2006 Jun;106(12):2682-2691.
- 4. Dickey ID, Rose PS, Fuchs B, Wold LE, Okuno SH, Sim FH, et al. Dedifferentiated chondrosarcoma: the role of chemotherapy with updated outcomes. J Bone Joint Surg Am 2004 Nov;86(11):2412-2418.
- 5. Grimer RJ, Gosheger G, Taminiau A, Biau D, Matejovsky Z, Kollender Y, et al. Dedifferentiated chondrosarcoma: prognostic factors and outcome from a European group. Eur J Cancer 2007 Sep;43(14):2060-2065.
- 6. Kawaguchi S, Sun T, Lin PP, Deavers M, Harun N, Lewis VO. Does ifosfamide therapy improve survival of patients with dedifferentiated chondrosarcoma? Clin Orthop Relat Res 2014 Mar;472(3):983-989.
- 7. Angelini A, Guerra G, Mavrogenis AF, Pala E, Picci P, Ruggieri P. Clinical outcome of central conventional chondrosarcoma. J Surg Oncol 2012 Dec;106(8):929-937.
- 8. Lee FY, Mankin HJ, Fondren G, Gebhardt MC, Springfield DS, Rosenberg AE, et al. Chondrosarcoma of bone: an assessment of outcome. J Bone Joint Surg Am 1999 Mar;81(3):326-338.
- 9. Zeifang F, Buchner M, Zahlten-Hinguranage A, Bernd L, Sabo D. Complications following operative treatment of primary malignant bone tumours in the pelvis. Eur J Surg Oncol 2004 Oct;30(8):893-899.
- 10. Enneking WF, Dunham WK. Resection and reconstruction for primary neoplasms involving the innominate bone. J Bone Joint Surg Am 1978 Sep;60(6):731-746.
- 11. Konishi E, Nakashima Y, Mano M, Tomita Y, Nagasaki I, Kubo T, et al. Primary central chondrosarcoma of long bone, limb girdle and trunk: Analysis of 174 cases by numerical scoring on histology. Pathol Int 2015 Sep;65(9):468-475.
- 12. Bindiganavile S, Han I, Yun JY, Kim H-S. Long-term outcome of chondrosarcoma: a single institutional experience. Cancer research and treatment: Official Journal of Korean Cancer Association 2015;47(4):897.
- 13. Bederman SS, Shah KN, Hassan JM, Hoang BH, Kiester PD, Bhatia NN. Surgical techniques for spinopelvic reconstruction following total sacrectomy: a systematic review. Eur Spine J 2014 Feb;23(2):305-319.
- 14. Italiano A, Mir O, Cioffi A, Palmerini E, Piperno-Neumann S, Perrin C, et al. Advanced chondrosarcomas: role of chemotherapy and survival. Ann Oncol 2013 Nov;24(11):2916-2922.
- 15. Liang Z, Brooks J, Willard M, Liang K, Yoon Y, Kang S, et al. CXCR4/CXCL12 axis promotes VEGF-mediated tumor angiogenesis through Akt signaling pathway. Biochem Biophys Res Commun 2007 Aug;359(3):716-722.
- 16. Sun X, Charbonneau C, Wei L, Yang W, Chen Q, Terek RM. CXCR4-targeted therapy inhibits VEGF expression and chondrosarcoma angiogenesis and metastasis. Mol Cancer Ther 2013 Jul;12(7):1163-1170.
- 17. Onishi AC, Hincker AM, Lee FY. Surmounting chemotherapy and radioresistance in chondrosarcoma: molecular mechanisms and therapeutic targets. Sarcoma 2010;2011.
- 18. Yin H, Zhou W, Meng J, Zhang D, Wu Z, Wang T, et al. Prognostic factors of patients with spinal chondrosarcoma: a retrospective analysis of 98 consecutive patients in a single center. Ann Surg Oncol 2014 Oct;21(11):3572-3578.
- 19. Kim HS, Bindiganavile SS, Han I. Oncologic outcome after local recurrence of chondrosarcoma: analysis of prognostic factors. J Surg Oncol 2015 Jun;111(8):957-961.
- 20. Outani H, Hamada K, Imura Y, Oshima K, Sotobori T, Demizu Y, et al. Comparison of clinical and functional outcome between surgical treatment and carbon ion radiotherapy for pelvic chondrosarcoma. Int J Clin Oncol 2016 Feb;21(1):186-193.
- 21. Uhl M, Mattke M, Welzel T, Oelmann J, Habl G, Jensen AD, et al. High control rate in patients with chondrosarcoma of the skull base after carbon ion therapy: first report of long-term results. Cancer 2014 May;120(10):1579-1585.
- 22. Roos E, van Coevorden F, Verhoef C, Wouters MW, Kroon HM, Hogendoorn PC, et al. Prognosis of primary and recurrent chondrosarcoma of the rib. Ann Surg Oncol 2016 Mar;23(3):811-817.
- 23. Al-Refaie RE, Amer S, Ismail MF, Al-Shabrawy M, Al-Gamal G, Mokbel E. Chondrosarcoma of the chest wall: single-center experience. Asian Cardiovasc Thorac Ann 2014 Sep;22(7):829-834.
- 24. Ogose A, Unni KK, Swee RG, May GK, Rowland CM, Sim FH. Chondrosarcoma of small bones of the hands and feet. Cancer 1997 Jul;80(1):50-59.
- 25. Widhe B, Bauer HC; Scandinavian Sarcoma Group. Surgical treatment is decisive for outcome in chondrosarcoma of the chest wall: a population-based Scandinavian Sarcoma Group study of 106 patients. J Thorac Cardiovasc Surg 2009 Mar;137(3):610-614.
- 26. Strotman PK, Reif TJ, Kliethermes SA, Sandhu JK, Nystrom LM. Dedifferentiated chondrosarcoma: a survival analysis of 159 cases from the SEER database (2001-2011). J Surg Oncol 2017 Aug;116(2):252-257.