Non-alcoholic fatty liver disease (NAFLD) is a new public health problem and a complication associated with diabetes and metabolic syndrome.1 The defining feature of NAFLD is excess fat deposition on liver cells (hepatocytes), which may be accompanied by evidence of cell injury with or without the presence of fibrosis and inflammation non-alcoholic steatohepatitis (NASH) or rarely remains as an isolated event (non-alcoholic fatty liver, NAFL).2,3 The importance of recognizing this liver condition lies in the fact that it will overtake hepatitis C infection in the near future as the leading cause of liver failure and the need for transplantation in many developed countries, as well as the absence of FDA-approved therapies for this disease, thereby making the early detection or better still its prevention as an urgent healthcare agenda.4–6
As the pathogenesis of type 2 diabetes mellitus (T2DM) or insulin resistance is closely associated with the presence of NASH/NAFLD, the use of various antidiabetic drugs such as pioglitazone, metformin, dipeptidyl peptidase-4 inhibiter, and glucagon-like peptidase-1 agonists have been postulated to reduce hepatic inflammation in these liver conditions.7–10 Despite many studies, there is a lack of effective treatment for NAFLD/NASH.11 Sodium-glucose cotransporter 2 inhibitors (SGLT2Is) has revolutionized the treatment of T2DM with a unique mechanism of action and efficacy in reducing the glycated hemoglobin (HbA1c) levels. It acts by helping in renal excretion of glucose and, therefore, will cause a reduction of body weight (on average 2.5–3.0 kg) and prevalence of obesity that may improve the liver histology of those with NAFLD/NASH.12 Drugs in this class includes canagliflozin, dapagliflozin, empagliflozin, ertugliflozin, ipragliflozin, luseogliflozin and tofogliflozin. It can reduce the HbA1c by up to 0.8% and gain a foothold as one of the first-line antidiabetic drugs. Modest blood pressure reduction has also been documented together with a lower risk of hypoglycemia using these drugs.13 Furthermore, it is also effective in preventing weight gain.14
A systematic review published in 2019 summarized its finding based on eight studies 7,15–21 that showed a significant decrease in alanine transaminase (ALT), and reduction in aspartate transaminase (AST), and gamma-glutamyl transpeptidase (GGT) levels with the use of SGLT2Is.22 Several randomized controlled trials (RCTs) have been recently published that explored its benefits in improving liver functions.7,18,23–30 However, there is a lack of systematic review coupled with meta-analysis and trial sequential analysis (TSA) conducted to estimate the effect of SGLT2Is on hepatic enzymes among patients with diabetes. Meta-analysis can provide information on the threshold of statistical significance for weight mean differences. TSA will confirm the result from meta-analysis with a cumulative sample size of all included studies, thus reducing the chance for type 1 error due to systematic error or small sample size effect that could occur in a meta-analysis.
We sought to look at the efficacy of SGLT2Is compared to other antidiabetic drugs in improving the liver function parameters in T2DM patients with NAFLD. As a secondary objective, we will also perform a meta-analysis on changes in insulin resistance, glycemic and lipid parameters using SGLT2Is in these groups of patients.
Methods
The present systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).31 The protocol was registered in the PROSPERO (Record ID: 126327).
A systematic literature search was performed using three databases, Medline, Cochrane, and Embase, from inception to 20 October 2018. Searches were conducted using Medical Subject Headings terms and corresponding keywords, as shown in Appendix 1.
There were no language or method restrictions, and the eligibility criteria extend to all studies done globally [Figure 1]. Inclusion criteria are RCTs conducted on T2DM patients with NAFLD on treatment with antidiabetic drugs, namely SGLT2Is, along with its effects on NAFLD/NASH. Exclusion criteria will be any other study design such as review articles, prevalence studies, or animal and cells models.
The treatment or intervention group will be patients who are on SGLT2I treatment. The comparator or control group will be placebo/patients who are not on treatment with SGLT2I. Therefore, the context studied will be patients with T2DM with underlying NAFLD who are randomized to be receiving SGLT2I treatment or other oral antidiabetic drugs.
The primary outcome for these meta-analyses was the changes in hepatic enzymes levels, namely ALT, AST, and GGT. In addition, we also assessed the effect of SGLT2Is on insulin resistance and glycemic and lipid parameters such as triglyceride and cholesterol components.
Articles screening and data extraction was done through a multi-step process. Three independent authors preliminarily screened articles by their titles and abstracts, followed by full-text reading. This was followed by data extraction on the following aspects: primary author, year of publication, study country, sample size of the two groups and levels of liver enzymes level for ALT, AST, and GGT that was available for each of the selected articles. Finally, a standardized data extraction form was created, and the extracted data was inserted into this form. Any disagreement was discussed together with the following authors: ID, FKH, SMC, and SKV.
We used mean±standard deviation (SD) to express our outcomes. If the mean difference and SD were not provided, the mean was calculated by subtracting the mean of baseline measurement from the corresponding mean of post-intervention measurement, while the SD was imputed from the endpoint measurement. If the mean difference was provided, but the SD was not, the latter was imputed either from the endpoint measurement or calculated using the confidence intervals (CIs) with the following formula in Excel - “SQRT (sample size)*(upper CI-lower CI)/(T.INV.2T (0.05, $D$2-1)*2)”.32
Data for this study was extracted from the RCT studies and meta-analysis (random-effects model) was performed to estimate the pooled risk ratio at 95% CI based on the determination of heterogeneity among these studies by I2 statistics. In addition, TSA was performed to assess the effect of SGLT2I on NAFLD compared to the control group.33 The comparative effectiveness of SGLT2I was also studied using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach, which was not done in previous systematic reviews. This was done to rate the evidence’s quality as either high, moderate, low, or very low.
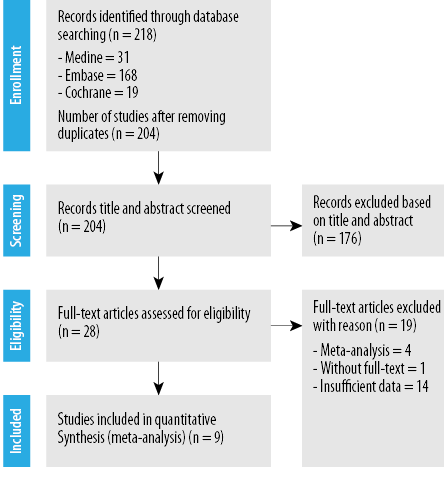
Figure 1: Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram of the literature screening process.
Included trials were independently assessed using the Revised Cochrane Risk of Bias Tool (RoB 2.0). Two authors independently assessed all trials identified for study inclusion after full-text reading (KWL and NKD). Any discrepancies were discussed with the following authors once again (MJS, ID, FKH, SMC, and SKV). Assessment was done across the five domains of bias (bias arising from the randomization process, bias due to deviations from intended interventions, bias due to missing outcome data, bias in measurement of the outcome, and bias in selection of the reported result).34 GRADE assessments were performed to appraise the quality of the evidence35 which assessed the studies inconsistency, indirectness, imprecision, and publication bias.36–37
Results
Through our initial search, we identified 218 eligible manuscripts [Figure 1]. After further de-duplication (n = 14), 204 studies were then selected for the next screening step. Through a review of the abstract, title, and keywords, eight studies were finally included, and its characteristics extracted as described in Table 1 and appendices 2a–c. These data were described based on the author’s name, antidiabetic drug used, and improvement in liver function as measured by the liver enzyme levels.
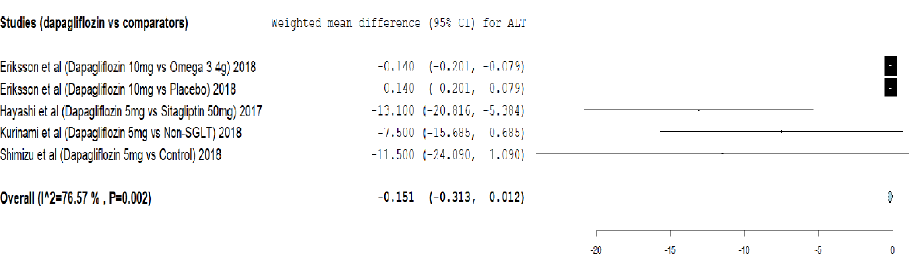
Figure 2: Meta-analysis on the effect of dapagliflozin versus comparators on alanine transaminase (ALT) reduction.
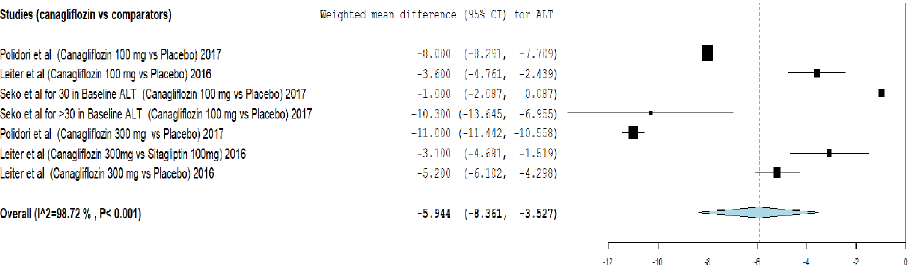
Figure 3: Meta-analysis on the effect of canagliflozin versus comparators on alanine transaminase (ALT) reduction.

Figure 4: Meta-analysis on the effect of dapagliflozin versus comparators on aspartate transaminase (AST) reduction.
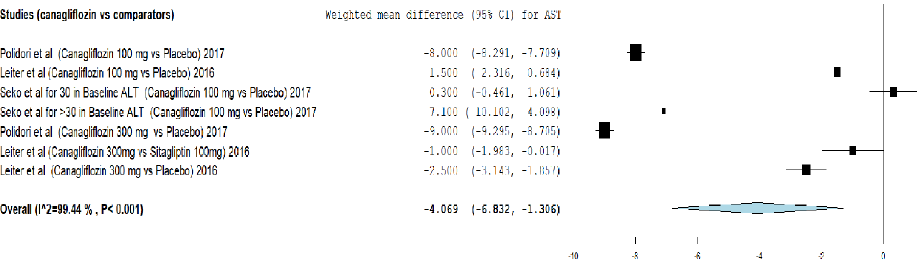
Figure 5: Meta-analysis on the effect of canagliflozin versus comparators on aspartate transaminase (AST) reduction.
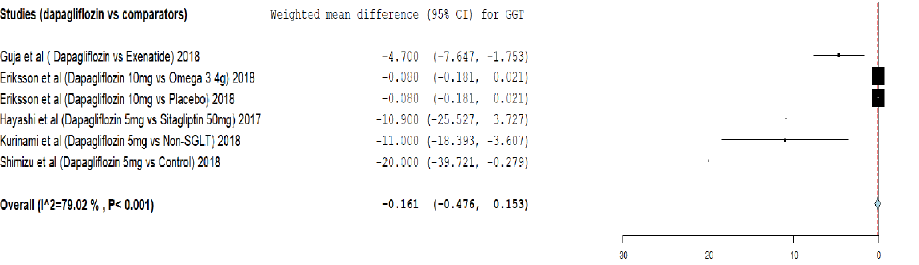
Figure 6: Meta-analysis on the effect of dapagliflozin versus comparators on gamma-glutamyl transpeptidase (GGT) reduction.
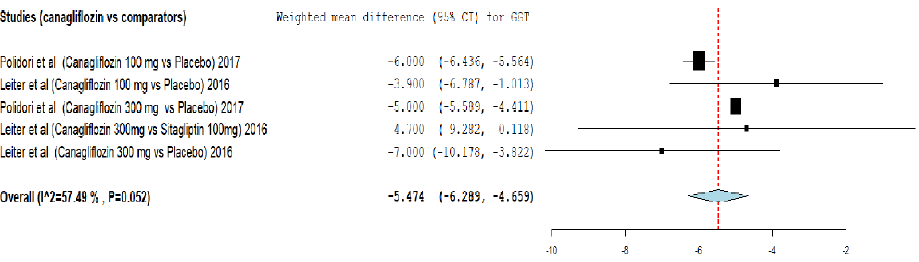
Figure 7: Meta-analysis on the effect of canagliflozin versus comparators on gamma-glutamyl transpeptidase (GGT) reduction.
Table 1 shows the key characteristics of the included studies, and appendices 2a–c indicates changes in hepatic functions among T2DM patients. In the final analysis, a total sample of 5984 patients with T2DM was included in which patients had used SGLT2Is in the treatment of their T2DM. The overall quality of included studies appeared to be good.
Table 1: Characteristics of included studies.
|
Eriksson et al,18 |
2018 |
Sweden |
Dapagliflozin 10 mg |
Omega-3 4 g |
Patients with T2DM (aged 40–75 years) |
Multi |
Double-blind |
2 |
12 weeks |
12 weeks |
|
Eriksson et al,18 |
2018 |
Sweden |
Dapagliflozin 10 mg |
Placebo |
Patients with T2DM |
Multi |
Double-blind |
2 |
12 weeks |
12 weeks |
|
Guja et al,23 |
2018 |
USA |
Dapagliflozin |
Exenatide |
Adults with T2DM and inadequate
glycemic control |
Multi |
Double-blind |
3 |
28 weeks |
28 weeks |
|
Hayashi et al,24 |
2017 |
Japan |
Dapagliflozin 5 mg |
Sitagliptin 50 mg |
Patients with T2DM taking prescribed oral hypoglycemic agents |
Single |
Open-label |
2 |
12 weeks |
12 weeks |
|
Kurinami et al,38 |
2018 |
Japan |
Dapagliflozin 5 mg |
Non-SGLT2 (din specify) |
Patients with T2DM |
Single |
Open-lable |
2 |
24 weeks |
24 weeks |
|
Leiter et al,27 |
2016 |
Germany |
Canagliflozin 300 mg |
Sitagliptin 100 mg |
Patients with T2DM |
Multi |
Double-blind |
3 |
26 weeks |
26 weeks |
|
Leiter et al,28 |
2016 |
Germany |
Canagliflozin 100 mg |
Placebo |
Patients with T2DM |
Multi |
Double-blind |
3 |
26 weeks |
26 weeks |
|
Leiter et al,28 |
2016 |
Germany |
Canagliflozin 300 mg |
Placebo |
Patients with T2DM |
Multi |
Double-blind |
3 |
26 weeks |
26 weeks |
|
Polidori et al,29 |
2017 |
USA |
Canagliflozin 100 mg |
Placebo |
Patients with T2DM |
Single |
Unknown |
3 |
26 weeks |
26 weeks |
|
Polidori et al,29 |
2017 |
USA |
Canagliflozin 300 mg |
Placebo |
Patients with T2DM |
Single |
Unknown |
3 |
26 weeks |
26 weeks |
|
Seko et al,7 |
2017 |
Japan |
Canagliflozin 100 mg |
Placebo |
Patients with T2DM
with Baseline ALT ≤ 30 |
Multi |
Double-blind |
3 |
12 weeks |
12 weeks |
|
Seko et al,7 |
2017 |
Japan |
Canagliflozin 100 mg |
Placebo |
Patients with T2DM
Baseline ALT > 30 |
Multi |
Double-blind |
3 |
12 weeks |
12 weeks |
RCT: randomized controlled trial; T2DM: type 2 diabetes mellitus; non-alcoholic fatty liver disease.
Table 2: Pooled weighted mean difference and 95% confidence interval of indicators for insulin resistance, glycemia, and lipid parameters between dapagliflozin and comparators.
|
Subcutaneous adipose tissue, cm2 |
4 |
-0.340 |
-0.814–0.133, |
86.1 |
< 0.001 |
|
Visceral adipose tissue, cm2 |
4 |
-0.316 |
-0.704–0.071, |
76.4 |
0.005 |
|
HbA1c, % |
5 |
-0.732 |
-1.087–-0.378 |
76.5 |
0.002 |
|
HOMA-IR |
3 |
-0.804 |
-1.336–-0.272, |
0.0 |
0.574 |
|
Serum triglyceride, mmol/L |
5 |
0.113 |
-0.278–0.504, |
76.6 |
0.002 |
|
Total cholesterol, mmol/L |
4 |
0.028 |
-0.223–0.279, |
25.1 |
0.261 |
|
Low-density lipoprotein, mmol/L |
5 |
0.118 |
-0.042–0.277, |
0.0 |
0.873 |
|
High-density lipoprotein, mmol/L |
5 |
0.034 |
-0.076–0.144, |
81.2 |
< 0.001 |
HBA1c: glycated hemoglobin; HOMA-IR: homeostatic model assessment for insulin sensitivity.
Analysis of the effect of dapagliflozin on ALT reduction using meta-analysis and TSA are provided in Figure 2 and Appendix 3a. The meta-analysis showed that dapagliflozin did not significantly reduce the ALT level by weighted mean difference (-0.151, 95% CI: -0.313–0.012) compared to other comparators. Moreover, the cumulative Z-curve (blue curve) did not cross the conventional boundary (Z-statistic > 1.96) and demonstrated that dapagliflozin did not significantly reduce ALT using the TSA. However, the number of patients included in the TSA did not exceed the required information size (i.e., 602 patients), indicating that the cumulative evidence for dapagliflozin not reducing ALT remains inconclusive based on only 266 patients.
Analysis of the effect of canagliflozin on ALT reduction using meta-analysis and TSA are provided in Figure 3 and Appendix 3b. The meta-analysis showed that canagliflozin significantly reduced the ALT level by weighted mean difference (-5.944, 95% CI: -8.361–-3.527) compared to other comparators. The cumulative Z-curve (blue curve) crossed the conventional boundary (Z-statistic above 1.96) and demonstrated that canagliflozin significantly reduced ALT using TSA. However, the number of patients included in the TSA did not exceed the required information size (5364 patients), indicating that the cumulative evidence is still inconclusive.
Analysis of the effect of dapagliflozin on AST reduction using meta-analysis and TSA are provided in Figure 4 and Appendix 3c. The meta-analysis showed that dapagliflozin did not significantly reduce the AST level by weighted mean difference (-0.078, 95% CI: -0.184–0.029) compared to comparators. The cumulative Z-curve (blue curve) did not cross the conventional boundary (Z-statistic above 1.96) and demonstrated that dapagliflozin did not significantly reduce AST using TSA. However, the number of patients included in the TSA did not exceed the required information size (3178 patients), indicating that the cumulative evidence remains inconclusive based on the 266 patients.
Analysis of effect of canagliflozin on AST reduction using meta-analysis and TSA are provided in Figure 5 and Appendix 3d. The meta-analysis showed that canagliflozin significantly reduced the AST level by weighted mean difference (-4.069, 95% CI: -6.832–-1.306) compared to other comparators. Moreover, the cumulative Z-curve (blue curve) crossed the conventional boundary (Z-statistic above 1.96) and demonstrated that canagliflozin significantly reduced AST using TSA. However, the number of patients included did not exceed the required information size (7015 patients), indicating that the cumulative evidence remains inconclusive based on 5287 patients.
Analysis of effect of dapagliflozin on GGT reduction using meta-analysis and TSA are provided in Figure 6 and Appendix 3e. The meta-analysis showed that dapagliflozin did not significantly reduce the GGT level by weighted mean difference (-0.161, 95% CI: -0.476–0.153) compared to comparators. The cumulative Z-curve (blue curve) did not cross the conventional boundary (Z-statistic > 1.96) and demonstrated that dapagliflozin did not significantly reduce GGT using the TSA. However, the number of patients included in our meta-analysis did not exceed the required information size (3923 patients), indicating that the cumulative evidence remains inconclusive based on the 723 patients.
Analysis of effect of canagliflozin on GGT reduction using meta-analysis and TSA are provided in Figure 7 and Appendix 3f. The meta-analysis showed that canagliflozin significantly reduced the GGT level by weighted mean difference (-5.474, 95% CI: -6.289–-4.659) compared to other comparators. The cumulative Z-curve (blue curve) crossed the conventional boundary (Z-statistic > 1.96) and demonstrated that canagliflozin significantly reduced GGT using TSA. In addition, the number of patients included in TSA exceeded the required information size (1627 patients), indicating that the cumulative evidence is conclusive.
Table 2 summarized the results from meta-analysis for subcutaneous adipose tissue, visceral adipose tissue, HbA1c, homeostatic model assessment for insulin sensitivity (HOMA-IR), serum triglyceride, total cholesterol, low-density lipoprotein (LDL), high-density lipoprotein (HDL), and adiponectin between dapagliflozin versus comparators. Based on the analysis, dapagliflozin statistical significantly reduced HbA1c and HOMA-IR by weight mean difference = -0.732 (95% CI: -1.087–-0.378) and -0.804 (95% CI: -1.336–0.272), respectively, compared to comparators. On the other hand, dapagliflozin had no statistically significant changes to subcutaneous adipose tissue, visceral adipose tissue, serum triglyceride, total cholesterol, LDL, HDL, and adiponectin.
Based on the data of included studies, Eriksson et al,18 reported that 33.3% of participants receiving dapagliflozin monotherapy experienced adverse events compared to placebo (28.6%), omega-3 monotherapy (40%), and dapagliflozin and omega-3 (68.2%).18 However, the authors did not mention what kind of adverse events were experienced by participants.
Seko et al,7 reported that 28.7% of participants in the high ALT and 33.4% of those with low ALT subgroups experienced adverse effects due to canagliflozin. There were no differences in the overall incidence of serious adverse events related to the canagliflozin between the high (1.0%) and low (0.3%) ALT subgroups. In addition, they also observed high and low ALT subgroup had a similar incidence of adverse events associated with symptomatic hypoglycemia, asymptomatic hypoglycemia, female genital infection, and osmotic diuresis, which were < 5%. One concern raised was that ketone bodies were significantly increased in both high and low ALT subgroups compared to placebo.7
Guja et al,23 2018; Hayashi et al,24 2017; Kurinami et al,38 2018; Leiter et al,28 2016; and Polidori et al,29 2017 did not report any adverse events from their studies.
Revised Cochrane Risk of Bias Tool assessment findings are given in appendices 4–5. The assessment indicated that two studies have a low risk of bias for all items,7,28 four studies had at least one item with unclear risk of bias,23,24,29,30 and three studies showed a high risk of bias.18,27,38 The high risk of bias was noted in the randomization process and deviation from the intended intervention in the study by Kurinami et al,38 2018 as well as bias due to missing outcome data in the study by Eriksson et al,18 2018.
GRADE assessment of the overall certainty of the evidence for the association between SGLT and hepatic enzyme levels reduction is presented in Appendix 6. Overall, the grade of evidence is low for the association between dapagliflozin and the reduction in hepatic enzymes levels and the association between canagliflozin and reduction of ALT and AST was also graded as low except for association between canagliflozin and GGT reduction which showed high certainty. These studies had to be downgraded for their inconsistency and imprecision.
Discussion
The present systematic review and meta-analysis of eight randomized controlled trials involved 5984 patients with T2DM. The analysis showed that canagliflozin reduced hepatic enzyme levels but not dapagliflozin. Based on TSA, we observed that the association between canagliflozin and the reduction in GGT is statistically significant, and this conclusive statement is drawn based on the total number of participants reaching the required sample size.
Our results support the use of canagliflozin but not dapagliflozin in the management of NASH/NAFLD as it has been shown to significantly reduced ALT, AST, and GGT as demonstrated in our meta-analysis. This is based on findings from Figures 3–7. This indicates another possible untapped use of canagliflozin in the treatment of NASH/NAFLD. This is in agreement with study by Leiter et al,28 which showed a similar reduction in ALT and AST levels with the use of canagliflozin. The study included four pools of patients: on canagliflozin alone, add-on to metformin, as an add-on to metformin and sulphonylurea, and add-on to metformin plus pioglitazone (i.e., without insulin).28 This indicates the wide range of the benefit of SGLT2Is that extends beyond any other antidiabetic drug that is used. The study also showed the effectiveness of canagliflozin in reducing GGT levels.28
As mentioned earlier, insulin resistance appears to the main link between T2DM and NAFLD/NASH, with additional contribution from obesity and other metabolic risk factors such as raised triglycerides and reduced HDL-C.39 There is increase transportation of free fatty acids to the liver due to insulin resistance, which diminishes the natural process of lipolysis by the now defunctioning insulin.2 As a secondary effect, this extra supply of fatty acid will drive the synthesis of triglycerides that is further stimulated by the recurring phenomenon of impaired hepatic fatty acid oxidation secondary to insulin resistance and the excess secretion of very LDL that will further worsen the fatty liver.3
The result in this study differs from the finding in a systematic review by Raj et al.22 The possible explanation for the difference could be due to the fact that the study by Raj et al,22 summarized the finding based on four RCTs15–18 and four observational studies7,19–21 compared to the nine RCTs in this study. Secondly, their finding was made based on small sample sizes, and the authors did not pool the sample size from each study examining the effect of SGLT, compared to our study that made its conclusion based on a pooled sample size of 5984 patients. Furthermore, Raj et al,22 did not perform any meta-analysis and TSA. Thus, the beneficial effect of SGLT may not be the true effect.
In addition, there may also be a strong molecular basis for the occurrence of NAFLD/NASH. This is based on the theory that carbon monoxide releasing molecule-A1 (CORM-A1) reduces damages to the liver tissue with steatosis via a dual action of improved mitochondrial function and nuclear factor-erythroid 2 related factor 2 activation.5 This may indicate that CORM-A1 has a huge potential of being an anti-NASH and anti-NAFLD agent.5 However, more researches need to be done on this exciting prospect before it is marketed as a treatment for NAFLD/NASH.
Some literature paradoxically noted that the inflammatory changes in NAFLD/NASH might, in turn, contribute to the development of T2DM that was thought to be mainly autoimmune in origin.6,40 Therefore, the relationship between both conditions associated with metabolic syndrome may be a two-way relationship. This opens up the hypothesis that curing NAFLD/NASH may improve hyperglycemia or even revert it totally to normoglycemia, thereby ending the decades of a long search for a cure for T2DM. Curing T2DM will go a low way in improving the health profile of many people worldwide, and that in turn will churn out more productivity to spur the world’s economy.
In addition, when looking at the effect of SGLT2Is on insulin resistance, glycemic, and lipid parameters, it was noted that, dapagliflozin significantly reduced HbA1c which is a parameter of glycemic control and HOMA-IR, which is a parameter of insulin sensitivity by weight mean difference = -0.732 (95% CI: -1.087–-0.378) and -0.804 (95% CI: -1.336–0.272), respectively, compared to comparators. This is expected as the primary action of SGLT2Is is in reducing renal tubular glucose reabsorption, which enables a reduction in HbA1c between 0.6–0.8%.41 SGLT2I can also improve insulin sensitivity via several molecular pathways, including beta function improvement, reduction of oxidative stress and inflammation, as well as disposition of calories and weight loss.42 However, there was no significant effect on lipid parameters such as the triglycerides and cholesterol components.
In a study in Japan, treatment of T2DM patients along with biopsy-proven NASH with dapagliflozin resulted in significant reductions in HbA1c, fasting glucose levels, and reduced visceral fat mass as early as four weeks treatment.43 Another Japanese study using serial liver biopsies in five patients receiving 24 weeks of canagliflozin showed remarkable NASH histology improvement.44 However, the number of subjects involved was relatively small, and more studies are needed to show a definite significant effect of hepatic fat reduction with SGLT2Is.
Future studies are recommended given the findings of this study to instill confidence in doctors in prescribing SGLT2Is in patients with NASH/NAFLD in view of the potential beneficial added effect in reducing ALT, AST, and GGT. This is to ensure that this drug is safe, effective, and accessible to patients with T2DM and manages to gain a foothold in many clinical practice guidelines on T2DM worldwide to encourage physicians to confidently prescribe it as a management option in patients with NASH/NAFLD.
The potential adverse events with SGLT2Is could be adverse cardiovascular events. Studies reported that dapagliflozin could lead to major adverse cardiovascular events45 and canagliflozin could cause genital tract infections and osmotic diuresis-related adverse events.46 Overall, there were no new or unexpected adverse events compared with previous studies with these treatments.
This is the first study of the effect of SGLT2Is on hepatic enzymes performed using meta-analysis with TSA to estimate the effect of SGLT2Is on hepatic enzymes. TSA provides the information on the power of sample size of cumulative meta-analysis and whether it surpasses the conventional and alpha spending boundaries, which indicates whether the evidence of our meta-analysis is statistically significant and conclusive or not. However, the current study has several limitations. Firstly, the majority of TSA indicated that the pooled sample size did not meet the required sample size for drawing the conclusive effect of SGLT2Is. Secondly, there are serious inconsistencies in the pooled weighted mean difference for ALT, AST, and GGT using dapagliflozin, and very serious inconsistencies in pooled weighted mean difference ALT and AST using canagliflozin. Thirdly, there is serious imprecision in pooled weighted mean difference for ALT, AST, and GGT using dapagliflozin. This could be due to low certainty. Fourthly, the majority of studies did not report data on changes in liver attenuation, liver-to-spleen attenuation ratio, liver magnetic resonance imaging proton density fat fraction, and liver fat volume; therefore, we could not assess the effect of SGLT2Is on hepatic fibrosis and hepatic fat content. Notwithstanding these limitations, this study suggests that more, higher quality randomized trials testing the effect of dapagliflozin and canagliflozin on hepatic enzyme levels reduction are needed to address these uncertainties and better understand the differences between SGLT2Is effectiveness. There is also a need for large randomized trials that assess more patients to make a conclusive statement.
TSA also shows that the evidence is still inconclusive for using these SGLT2Is to improve liver function parameters. Therefore, more studies are needed before any recommendations are made regarding using SGLT2Is as a treatment of NAFLD/NASH. However, with the results obtained from this study, promise holds that SGLT2Is may be the answer to the yet non-curative NAFLD/NASH.
Conclusion
Canagliflozin but not dapagliflozin is effective in improving ALT, AST, and GTT levels among patients with diabetes, suggesting they may be useful in managing diabetes with fatty liver.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
references
- 1. Saponaro C, Gaggini M, Gastaldelli A. Nonalcoholic fatty liver disease and type 2 diabetes: common pathophysiologic mechanisms. Curr Diab Rep 2015 Jun;15(6):607.
- 2. Wong VW, Wong GL, Choi PC, Chan AW, Li MK, Chan HY, et al. Disease progression of non-alcoholic fatty liver disease: a prospective study with paired liver biopsies at 3 years. Gut 2010 Jul;59(7):969-974.
- 3. Gramlich T, Kleiner DE, McCullough AJ, Matteoni CA, Boparai N, Younossi ZM. Pathologic features associated with fibrosis in nonalcoholic fatty liver disease. Hum Pathol 2004 Feb;35(2):196-199.
- 4. Oseini AM, Sanyal AJ. Therapies in non-alcoholic steatohepatitis (NASH). Liver Int 2017 Jan;37(Suppl 1):97-103.
- 5. Bondini S, Younossi ZM. Non-alcoholic fatty liver disease and hepatitis C infection. Minerva Gastroenterol Dietol 2006 Jun;52(2):135-143.
- 6. Sanyal AJ. Review article: non-alcoholic fatty liver disease and hepatitis C–risk factors and clinical implications. Aliment Pharmacol Ther 2005 Nov;22(Suppl 2):48-51.
- 7. Seko Y, Sumida Y, Tanaka S, Mori K, Taketani H, Ishiba H, et al. Effect of sodium glucose cotransporter 2 inhibitor on liver function tests in Japanese patients with non-alcoholic fatty liver disease and type 2 diabetes mellitus. Hepatol Res 2017 Sep;47(10):1072-1078.
- 8. Promrat K, Lutchman G, Uwaifo GI, Freedman RJ, Soza A, Heller T, et al. A pilot study of pioglitazone treatment for nonalcoholic steatohepatitis. Hepatology 2004 Jan;39(1):188-196.
- 9. Loomba R, Lutchman G, Kleiner DE, Ricks M, Feld JJ, Borg BB, et al. Clinical trial: pilot study of metformin for the treatment of non-alcoholic steatohepatitis. Aliment Pharmacol Ther 2009 Jan;29(2):172-182.
- 10. Iwasaki T, Yoneda M, Inamori M, Shirakawa J, Higurashi T, Maeda S, et al. Sitagliptin as a novel treatment agent for non-alcoholic Fatty liver disease patients with type 2 diabetes mellitus. Hepatogastroenterology 2011 Nov-Dec;58(112):2103-2105.
- 11. Hookman P, Barkin JS. Current biochemical studies of non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH) suggest a new therapeutic approach. Am J Gastroenterol 2003 Feb;98(2):495-499.
- 12. Brunton SA. The potential role of sodium glucose cotransporter 2 inhibitors in the early treatment of type 2 diabetes mellitus. Int J Clin Pract 2015 Oct;69(10):1071-1087.
- 13. Ministry of Health Malaysia. Clinical practice guidelines: management of type 2 diabetes mellitus. CPG Secretariat, Health Technology Assessment Section, Medical Development; 2015.
- 14. Fonseca VA. New developments in diabetes management: medications of the 21st century. Clin Ther 2014 Apr;36(4):477-484.
- 15. Kuchay MS, Krishan S, Mishra SK, Farooqui KJ, Singh MK, Wasir JS, et al. Effect of empagliflozin on liver fat in patients with type 2 diabetes and nonalcoholic fatty liver disease: a randomized controlled trial (E-LIFT Trial). Diabetes Care 2018 Aug;41(8):1801-1808.
- 16. Ito D, Shimizu S, Inoue K, Saito D, Yanagisawa M, Inukai K, et al. Comparison of ipragliflozin and pioglitazone effects on nonalcoholic fatty liver disease in patients with type 2 diabetes: a randomized, 24-week, open-label, active-controlled trial. Diabetes Care 2017 Oct;40(10):1364-1372.
- 17. Shibuya T, Fushimi N, Kawai M, Yoshida Y, Hachiya H, Ito S, et al. Luseogliflozin improves liver fat deposition compared to metformin in type 2 diabetes patients with non-alcoholic fatty liver disease: a prospective randomized controlled pilot study. Diabetes Obes Metab 2018 Feb;20(2):438-442.
- 18. Eriksson JW, Lundkvist P, Jansson P-A, Johansson L, Kvarnström M, Moris L, et al. Effects of dapagliflozin and n-3 carboxylic acids on non-alcoholic fatty liver disease in people with type 2 diabetes: a double-blind randomised placebo-controlled study. Diabetologia 2018 Sep;61(9):1923-1934.
- 19. Ohki T, Isogawa A, Toda N, Tagawa K. Effectiveness of ipragliflozin, a sodium-glucose cotransporter 2 inhibitor, as a second-line treatment for non-alcoholic fatty liver disease patients with type 2 diabetes mellitus who do not respond to incretin-based therapies including glucagon-like peptide-1 analogs and dipeptidyl peptidase-4 inhibitors. Clin Drug Investig 2016 Apr;36(4):313-319.
- 20. Gautam A, Agrawal PK, Doneria J, Nigam A. Effects of canagliflozin on abnormal liver function tests in patients of type 2 diabetes with non-alcoholic fatty liver disease. J Assoc Physicians India 2018 Aug;66(8):62-66.
- 21. Sumida Y, Murotani K, Saito M, Tamasawa A, Osonoi Y, Yoneda M, et al. Effect of luseogliflozin on hepatic fat content in type 2 diabetes patients with non-alcoholic fatty liver disease: A prospective, single-arm trial (LEAD trial). Hepatol Res 2019 Jan;49(1):64-71.
- 22. Raj H, Durgia H, Palui R, Kamalanathan S, Selvarajan S, Kar SS, et al. SGLT-2 inhibitors in non-alcoholic fatty liver disease patients with type 2 diabetes mellitus: a systematic review. World J Diabetes 2019 Feb;10(2):114-132.
- 23. Guja C, Repetto E, Han J, Hardy E, Jabbour SA. Effect of the exenatide plus dapagliflozin combination on fatty liver index and insulin resistance in type 2 diabetes patients: The DURATION-8 trial. Diabetologia 2018;61(Supplement 1):S350.
- 24. Hayashi T, Fukui T, Nakanishi N, Yamamoto S, Tomoyasu M, Osamura A, et al. Dapagliflozin decreases small dense low-density lipoprotein-cholesterol and increases high-density lipoprotein 2-cholesterol in patients with type 2 diabetes: comparison with sitagliptin. Cardiovascular Diabetology 2017;16(1):1-3.
- 25. Hayashi T, Yamamoto S, Tomoyasu M, Hara N, Ohara M, Yamamoto T, et al. Dapagliflozin increases large, buoyant Ldl but reduces small, dense LDL in patients with type 2 diabetes: Comparison with Sitagliptin. Diabetes 2016;65(Supplement 1):A161.
- 26. Kusunoki M, Natsume Y, Miyata T, Tsutsumi K, Oshida Y. Effects of concomitant administration of a dipeptidyl peptidase-4 inhibitor in Japanese patients with type 2 diabetes showing relatively good glycemic control under treatment with a sodium glucose cotransporter 2 inhibitor. Drug Research 2018;68(12):704-709.
- 27. Leiter LA, Forst T, Polidori D, Balis D, Xie J, Sha S, et al. Changes in liver function tests (LFTS) with canagliflozin (CANA) vs. Sitagliptin (SITA) in patients with type 2 diabetes mellitus (T2DM) are related to changes in A1c and body weight (BW). Diabetes 2015;64:A328.
- 28. Leiter LA, Forst T, Polidori D, Balis DA, Xie J, Sha S. Effect of canagliflozin on liver function tests in patients with type 2 diabetes. Diabetes Metab 2016 Feb;42(1):25-32.
- 29. Polidori D, Meininger G. Effect of canagliflozin (CANA) on liver function tests (LFTs) in patients with type 2 diabetes mellitus (T2DM) and presumed liver fibrosis suggestive of nonalcoholic steatohepatitis (NASH). Canadian Journal Diabetes 2017;41(5):S75.
- 30. Shimizu M, Suzuki K, Kato K, Jojima T, Iijima T, Murohisa T, et al. Evaluation of the effects of dapagliflozin, a sodium-glucose co-transporter-2 inhibitor, on hepatic steatosis and fibrosis using transient elastography in patients with type 2 diabetes and non-alcoholic fatty liver disease. Diabetes Obes Metab 2019:21(2):285-292.
- 31. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015 Jan;4(1):1-9.
- 32. Jenkins WL. A quick method for estimating standard deviation. Psychol Rep 1955;1(3):77-78.
- 33. TSA software. 0.9 beta ed. Copenhagen trial unit, centre for clinical intervention research, Copenhagen, Denmark; 2011.
- 34. Higgin J, Green S. (2011) Cochrane handbook for systematic reviews of interventional version 5.10. The Cochrane collaboration.
- 35. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al; GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008 Apr;336(7650):924-926.
- 36. Guyatt GH, Oxman AD, Kunz R, Atkins D, Brozek J, Vist G, et al. GRADE guidelines: 2. Framing the question and deciding on important outcomes. J Clin Epidemiol 2011 Apr;64(4):395-400.
- 37. Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 2011 Apr;64(4):401-406.
- 38. Kurinami N, Sugiyama S, Yoshida A, Hieshima K, Miyamoto F, Kajiwara K, et al. Dapagliflozin significantly reduced liver fat accumulation associated with a decrease in abdominal subcutaneous fat in patients with inadequately controlled type 2 diabetes mellitus. Diabetes Res Clin Pract 2018 Aug;142:254-263.
- 39. Neuschwander-Tetri BA. Non-alcoholic fatty liver disease. BMC Med 2017 Feb;15(1):45.
- 40. Farrell GC, Wong VW, Chitturi S. NAFLD in Asia–as common and important as in the west. Nat Rev Gastroenterol Hepatol 2013 May;10(5):307-318.
- 41. Hsia DS, Grove O, Cefalu WT. An update on SGLT2 inhibitors for the treatment of diabetes mellitus. Curr Opin Endocrinol Diabetes Obes 2017;24:73.
- 42. Yaribeygi H, Sathyapalan T, Maleki M, Jamialahmadi T, Sahebkar A. Molecular mechanisms by which SGLT2 inhibitors can induce insulin sensitivity in diabetic milieu: a mechanistic review. Life Sci 2020 Jan;240:117090.
- 43. Tobita H, Sato S, Miyake T, Ishihara S, Kinoshita Y. Effects of dapagliflozin on body composition and liver tests in patients with nonalcoholic steatohepatitis associated with type 2 diabetes mellitus: a prospective, open-label, uncontrolled study. Curr Ther Res Clin Exp 2017 Jul;87:13-19.
- 44. Akuta N, Watanabe C, Kawamura Y, Arase Y, Saitoh S, Fujiyama S, et al. Effects of a sodium-glucose cotransporter 2 inhibitor in nonalcoholic fatty liver disease complicated by diabetes mellitus: preliminary prospective study based on serial liver biopsies. Hepatol Commun 2017 Feb;1(1):46-52.
- 45. Sonesson C, Johansson PA, Johnsson E, Gause-Nilsson I. Cardiovascular effects of dapagliflozin in patients with type 2 diabetes and different risk categories: a meta-analysis. Cardiovasc Diabetol 2016 Feb;15:37.
- 46. Yang X-P, Lai D, Zhong X-Y, Shen H-P, Huang Y-L. Efficacy and safety of canagliflozin in subjects with type 2 diabetes: systematic review and meta-analysis. Eur J Clin Pharmacol 2014 Oct;70(10):1149-1158.