Type 2 diabetes mellitus (T2DM) and obesity are prevalent in the UAE.1 Genetic susceptibility and lifestyle factors, mainly lack of healthy food and good exercise, contribute to the pathogenesis of these coupled disorders.2–4 Thus, preventive strategies are needed. One thoughtful approach would be to develop culturally fitting national education programs that promote a healthy diet and physical activity/exercise.
The chance of developing T2DM is about two-fold higher in people with a positive family history of the disease.3 Early interventions (e.g., 5–7% decrease in weight) have been shown to produce measurable health benefits, including reducing the rate of developing diabetes.5 Thus, it is pivotal to improve public awareness of 1) rising rates of obesity, diabetes, and cardiovascular disease, 2) mechanisms underlying susceptibility to these disorders, and 3) the ability to ameliorate risks of obesity, diabetes, and cardiovascular disease by proper diet and physical activity/exercise.
While DM continues to become a global public health emergency, and despite several randomized clinical trials confirming the beneficial role of lifestyle modification in the prevention of DM, currently, there are hardly any national programs in the Middle East and North Africa region for the prevention of DM.6
Structured educational programs (addressing family risk information and strategies to change lifestyles) that target first-degree relatives of T2DM patients have not been previously developed and implemented in the UAE. In this study, we used the individualized, family-based interactive lifestyle education program ‘DiAlert for individuals with a positive family history of diabetes’.7 DiAlert is a low-intensive, structured, theory-based lifestyle education program developed to target overweight first-degree relatives of patients with T2DM. The program aims to help them reduce their risk of diabetes and related cardiovascular disease.7,8
Our study sought to investigate the feasibility and impact of this education program in a sample of adult Emiratis with overweight or obesity and a positive family history of T2DM. Specifically, the study aimed to assess the differences on the outcomes (waist circumference, weight, body mass index (BMI), blood pressure, and diabetes and related cardiovascular biomarkers) between the baseline, visit one (after three months) and visit two (after six months). Using a convenient sample, the present feasibility study will allow us to strengthen the methodology and the implementation of the DiAlert program in the UAE.
Methods
This is a pilot study using a pre-post design without a control arm, carried out at the Diabetes Center at Tawam Hospital in Al Ain, UAE. The study was performed in collaboration with the College of Medicine and Health Sciences of the UAE University (Al Ain, UAE) and the VU University Medical Center – EMGO Institute for Health and Care Research (Amsterdam, Netherlands). The Research Ethics Committee of the Al Ain Medical District Human Research Ethics Committee (protocol No. 14/09, CRD 312/14) approved the study. All participants provided written informed consent before study enrolment.
We aimed to include about 30 Emiratis, which would allow us to test the acceptability, feasibility, and effects of DiAlert in the UAE. Inclusion criteria were people with overweight or obesity, between 18 and 55 years old, and having one parent with T2DM. Exclusion criteria were people previously diagnosed with diabetes mellitus, pregnant women, those with serious medical or psychiatric morbidity, using medications that might affect weight, enrollment in another study, and illiterate. The study was conducted close to participants’ homes to reduce any potential barriers.
Recruitment was based on advertisements (flyers) and diabetic patients’ recommendations to their relatives and friends. Study assessments were performed at baseline, three months, and six months. All participants completed culturally validated questionnaires on sociodemographics, risk perception, lifestyle (diet, physical activity, and smoking), outcome expectancy, and self-efficacy. Anthropometric and laboratory measurements were performed as described herein.
Health counselors, diabetes educators, dieticians, and endocrinologists were trained to deliver the Arabic version of DiAlert during three planned visits. The information offered insights into diabetes prevention, lifestyle changes, and motivational skills (personal stories, motivational techniques, interactive group education, and action planning).7,9,10 The Dutch manual and materials were translated into English and forward translated into Arabic. A Dutch physician fluent in Arabic checked the version and provided feedback. Experts and laypersons were also asked by the project steering committee to comment on the study materials. Study manuals, questionnaires, and materials were then finalized with particular attention to cultural aspects (no ‘deep structure’ changes were deemed necessary). The educators delivering DiAlert in Arabic received a trainer manual and a local one-day workshop supervised by the Virje University Medical Center Amsterdam team. Medical students assisted the project steering committee in choosing text messages on health education (including video clips and audio tapes), and reminders were sent three times per week.
All participants were assigned to three interactive education sessions, one at the start of the study (baseline), one at three months, and one at six months. These sessions were individualized or group-based according to the patient’s choice. Most participants preferred the individualized sessions in the presence of family members, where feasible. Two trained diabetes educators and a dietician conducted the training sessions. Each session lasted between 60 to 90 minutes, and its contents included eliciting personal views on T2DM risk factors, prevention opportunities, energy balance, taking control (nutrition and exercise), and formulating an action plan to change lifestyle (diet/exercise). The sessions were interactive, allowing participants to pose questions and engage in developing and reviewing their action plans.
We also used a questionnaire to assess beliefs and risk perceptions, commitment, and nutritional choices of the participants. The questionnaire was based on a Likert scale score between 0 and 2, where 0 was do not agree, 1 was agree, and 2 was strongly agree. The beliefs and risk perceptions sections used this score to rate the following sentences: 1) diabetes is hereditary; 2) diabetes is caused by diet and eating habits; 3) diabetes is caused by doing little exercise; 4) diabetes is caused by being overweight; 5) a person can reduce the possible risk of getting diabetes; 6) personal efforts control the risk of getting diabetes; 7) I can control the risk of getting diabetes; 8) people who make good efforts are much less likely to get diabetes; 9) I have control over the risk of getting diabetes; and 10) diabetes is not caused by chance or bad luck.
We used the same Likert scale to score the following statements to understand the participants’ commitment (intention) to a healthy lifestyle: 1) changing my diet in the coming weeks and months; 2) stick to a healthy diet; 3) change to a physically active lifestyle; 4) exercise regularly in the coming weeks and months; 5) lose weight in the coming weeks; and 6) lose weight in the coming months.
Self-reports of nutritional choices (carbohydrate and fat consumption) were also measured using a Likert scale score between 0 to 2, where 0 was at least three times per week, 1 was less than three times per week, and 2 was never or less than once a month. The participants scored the frequency of their nutritional choices answering the question: “Over the past months, how often did you eat or drink” 1) eggs fried in oil, 2) fresh juice, 3) ready-made juice, 4) fruits, 5) dried fruits/dates, 6) cheese/cheese spread, 7) French fries/home fries, 8) potatoes, 9) salad dressing, 10) Biryani (rice), 11) meat, 12) harees (beaten wheat and meat), 13) luqaimat (sweet dumplings), 14) honey, and 15) Rgag (bread) with ghee.
A portable digital scale and stadiometer were used to measure weight and height. The measurements were rounded to the nearest 0.1 kg and 0.1 cm, respectively. Patients were asked to stand straight with their heads, backs, and buttocks vertically aligned to the height gauge.
Overweight was defined as a BMI of 25.0–29.9 kg/m2, and obesity as a BMI ≥ 30.0 kg/m2. Waist circumference was measured with an un-stretched tape midpoint between the bottom of the rib cage and the tip of the iliac crest. Blood pressure (systolic and phase-V diastolic) measurements were done on the right arm with an appropriate cuff size, in a sitting position (after 10 minutes of resting), using validated electronic sphygmomanometers (Omron Hem 907, Omron Healthcare, Kyoto, Japan), and the mean of three separate readings
was recorded.11,12
25-Hydroxyvitamin D, fasting blood glucose, high-density lipoprotein cholesterol (HDL), low-density lipoprotein cholesterol (LDL), and triglycerides (TG) were analyzed by COBAS INTEGRA® 400 Plus (Roche Diagnostics Corp., Indianapolis, USA). Serum high sensitivity C-reactive protein (hsCRP) was measured using the Synchron® Clinical System (UniCel DxC 800 from Beckman Coulter, Inc. Fullerton, CA, USA). Adiponectin (Human Total Adiponectin/Acrp30 Quantikine, DRP300), interleukin-6 (IL-6) (Human IL-6 Quantikine HS, HS600B), and tumor necrosis factor-alpha (TNF-α) (human TNF-alpha Quantikine, DTA00C) were measured by enzyme-linked immunosorbent assay following the manufacturer’s protocols.
Physical activity was objectively measured with accelerometers (190 Actigraph GT3X+). Accelerations were derived from the participant’s movements and converted into counts per minute. Participants were asked to wear the accelerometer attached to the right hip with an adjustable elastic waist belt for at least five days at the baseline, three months, and six months. Participants were asked to remove the accelerometer while swimming, showering, or bathing. Periods with no counts for ≥ 60 min were set as non-wear time. Days with
< 480 min activity were considered invalid, and the data excluded. Counts were analyzed as follows: sedentary < 100 counts/min; light 100–951 count/min; moderate 952–5724 counts/min; and vigorous activity > 5724 counts/min.
Before analyzing the subjects’ samples, the laboratory performed internal quality controls and participated in the External Quality Assurance program through the College of American Pathologists Proficiency Testing.
Statistical analyses were performed using SPSS Statistics 21.0 (IBM Corp. Released 2012. IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM Corp.). The analysis included baseline and subsequent visits data, without adjustments for confounders. Fixed effects repeated measures analysis of variance were done to compare variable changes with time, where responses at the baseline and two visits were assumed to have a compound symmetry correlation. The goodness-of-fit of the compound symmetry model was tested using the likelihood ratio test. Waist circumference was used in the post-hoc analysis, where participants with ≥ 2 cm reduction at visit two (six months) were considered ‘responders’ to the diabetes education program. This arbitrary (post-hoc) cut-off aimed to identify individuals who profited from the program versus those that did not. Confidence intervals and p-values are reported, although due to the study’s nature (feasibility study), p-values must be used wisely. A p-value < 0.050 was considered significant. The analysis plan presented will allow the study to prove its feasibility for implementing the Diabetes Educational Program in the referred context.
Results
One hundred twenty-two subjects responded to the announcement and were invited to be screened for eligibility. Every potential participant was given standardized written material, and the same nurse investigator explained the study protocol to minimize selection bias. Forty-four subjects (36.1%) who met the inclusion criteria agreed to participate and were enrolled in this six-month longitudinal study. Twelve of the 44 individuals (27.3%) dropped out after initial screening [Figure 1]. The final study number was 32 participants, and the retention rate was 72.7% for all three visits.
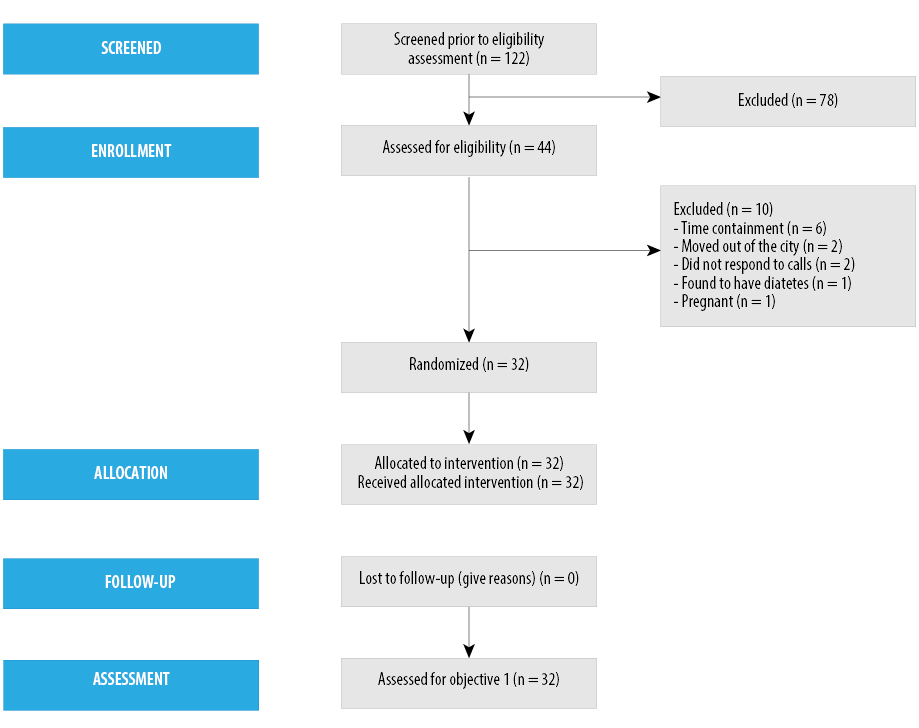
Figure 1: Participant flow.
The mean age of the participants was 35.0±9 years old, and 75.0% were females [Table 1]. Nine (28.1%) participants were obese, and 23 (71.9%) were overweight. Most participants (71.9%) had a university education, and more than half (56.3%) had two parents with T2DM. Ten (31.3%) participants reported dyslipidemia, making it the top comorbidity. One participant refused to measure their waist circumference.
The three consecutive interactive educational sessions were well attended and, for pragmatic reasons, were coupled with clinical and questionnaire assessments. The majority of sessions (80.0%) were delivered individually. In the 20.0% of group sessions, one or two family members were present.
Table 1: Baseline characteristics of the participants (n = 32).
|
Age, years |
35.0 ± 9.0 |
|
Female |
(75.0) |
|
Body mass index, kg/m2 |
|
|
Obese |
9 (28.1) |
|
Overweight |
23 (71.9) |
|
Education |
|
|
High school |
9 (28.1) |
|
University level |
23 (71.9) |
|
Parents with a history of T2DM |
|
|
One parent |
14 (43.8) |
|
Both parents |
18 (56.3) |
|
Comorbidities |
|
|
Dyslipidemia |
10 (31.3) |
|
Hypertension |
8 (25.0) |
|
Heart disease |
2 (6.3) |
T2DM: type 2 diabetes mellitus.
Twenty participants (64.5%) had decreased waist circumference ≥ 2 cm at three months, and 16 (51.6%) had decreased waist circumference ≥ 2 cm at six months [Figure 2]. Twenty (62.5%) participants had decreased weight at three months, and 18 (56.3%) participants had decreased weight at six months [Figure 2].
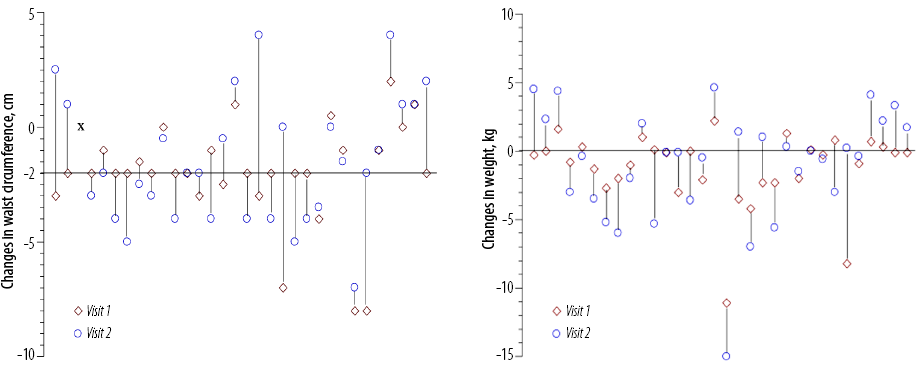
Figure 2: Changes in waist circumference and weight (baseline versus visit one and baseline versus visit two). Each vertical line represents data from each patient (n = 32). ‘x’ denotes the patient who refused waist circumference measurements. Left panel: Twenty (64.5%) of 31 participants had decreased waist circumference ≥ 2 cm at three months. Right panel: Twenty (62.5%) of 32 participants had decreased weight at three months.
Clinical and laboratory findings in all studied subjects over time are summarized in Table 2. Significant mean changes (baseline versus visit two) were observed in waist-circumference (p < 0.001), glycated hemoglobin level (HbA1c) (p = 0.007), HDL (p < 0.049), serum creatinine (p < 0.025), estimated glomerular filtration rate (eGFR) (p = 0.009), and adiponectin level (p < 0.024). In some variables, the confidence interval is not significant (i.e., there is no significant difference between baseline and visit two), while the p-value is significant. This may reflect a significant difference between visits one and two but not between baseline and visit two. No significant changes over time were observed in understanding diabetes risk factors and the role of lifestyle intention to adopt a healthy lifestyle and practicing healthy nutrition choices.
Baseline clinical and laboratory findings and changes over time (baseline versus visit two) in the ‘responders’ (decreased waist circumference ≥ 2 cm at visit two) and ‘non-responders’ (decreased waist circumference < 2 cm at visit two) are summarized in Table 3. Baseline fasting blood glucose, total cholesterol, TG, LDL were lower in the responders. Baseline adiponectin, HDL, vitamin D, average minutes per day with moderate-vigorous activity, average activity counts per day, TNF-α, and IL-6 were higher in the responders. In addition, more physical and laboratory improvements were noticed in responders versus non-responders; for example percent changes (baseline versus six months) in alanine transaminase (ALT) was -28.6 vs. +2.5; average minutes per day with moderate-vigorous activity was +66.1 vs. +7.4; average activity counts/day was +27.1 vs. -5.0; hsCRP was -34.9 vs. +22.4; TNF-α was -18.8 vs. +8.2; and adiponectin was +56.0 vs. +16.3.
Physical activity over time, based on accelerometer data (baseline, visit one, and visit two), are shown in Figure 3. Baseline average minutes per day with moderate and vigorous activity were 67.0% higher in responders than non-responders. Compared to baseline, at visit two, the average minutes per day with moderate and vigorous activity increased in the responders by 66.0% and in the non-responders by 7.0%. Similarly, at visit two (six months), the average activity counts per day increased in the responders by 27.0%, while it decreased in the non-responders.
Table 2: Clinical and laboratory findings in the studied subjects (n = 32).
|
Waist circumference, cm |
99.4 ± 11.9 |
97.3 ± 12.0 |
97.8 ± 12.2 |
0.7 |
2.5 |
< 0.001 |
-1.6 (± 0.4) |
|
Weight, kg |
86.1 ± 14.5 |
84.8 ± 14.2 |
85.1 ± 15.2 |
-0.2 |
2.2 |
0.101 |
-1.1 |
|
BMI, kg/m2 |
32.3 ± 4.4 |
31.9 ± 4.5 |
32.0 ± 4.9 |
-0.1 |
0.8 |
0.132 |
-1.1 |
|
Pulse rate, min |
82.0 ± 16.0 |
82.0 ± 13.0 |
79.0 ± 11.0 |
-2.2 |
8.1 |
0.473 |
-3.5 |
|
Systolic BP, mmHg |
124.0 ± 14.0 |
122.0 ± 13.0 |
121.0 ± 11.0 |
-2.0 |
7.5 |
0.521 |
-2.2 |
|
Diastolic BP, mmHg |
77.0 ± 11.0 |
77.0 ± 12.0 |
75.0 ± 11.0 |
-1.4 |
5.9 |
0.415 |
-2.9 |
|
Fasting blood glucose, mmol/L |
5.3 ± 0.7 |
5.3 ± 0.8 |
5.3 ± 0.5 |
-0.2 |
0.2 |
0.968 |
0.0 |
|
HbA1c, % |
5.6 ± 0.5 |
5.7 ± 0.6 |
5.5 ± 0.5 |
0.0 |
0.3 |
0.007 |
-2.3 (± 0.9) |
|
Total cholesterol, mmol/L |
5.0 ± 1.1 |
4.9 ± 1.2 |
5.0 ± 0.9 |
-0.1 |
0.3 |
0.172 |
0.0 |
|
Triglyceride, mmol/L |
1.3 ± 0.7 |
1.3 ± 0.7 |
1.3 ± 0.9 |
-0.2 |
0.2 |
0.905 |
0.0 |
|
HDL, mmol/L |
1.2 ± 0.3 |
1.3 ± 0.3 |
1.4 ± 0.4 |
-0.2 |
0.0 |
0.049 |
+8.9 (± 3.9) |
|
LDL, mmol/L |
3.1 ± 0.8 |
3.1 ± 0.6 |
3.1 ± 0.7 |
-0.2 |
0.2 |
0.901 |
0.0 |
|
ALT, unit/L |
30.8 ± 33.0 |
22.5 ± 14.0 |
26.3 ± 22.7 |
-4.5 |
12.5 |
0.563 |
-12.9 |
|
Urea, mmol/L |
3.2 ± 0.9 |
3.3 ± 0.8 |
3.4 ± 0.9 |
-0.5 |
0.1 |
0.476 |
+0.6 |
|
Creatinine, mmol/L |
63.5 ± 16.6 |
59.7 ± 18.2 |
61.8 ± 18.1 |
-1.2 |
4.2 |
0.025 |
-2.7 (± 2.5) |
|
eGFR, mL min-1/1.73 m2 |
112.6 ± 16.2 |
116.6 ± 16.2 |
112.6 ± 16.6 |
-3.1 |
2.4 |
0.009 |
-0.03 (±1.4) |
|
Vitamin D, nmol/L |
39.8 ± 24.9 |
44.8 ± 22.5 |
39.0 ± 20.7 |
-6.7 |
8.4 |
0.310 |
-2.0 |
|
hsCRP, mg/dL |
6.1 ± 8.6 |
5.5 ± 6.2 |
6.0 ± 5.4 |
-2.1 |
3.9 |
0.825 |
+37.0 |
|
ESR, mm/hr |
17.8 ± 10.5 |
17.7 ± 12.5 |
16.3 ± 11.4 |
-0.3 |
4.4 |
0.229 |
+11.0 |
|
TNF-α, pg/mL |
2.1 ± 1.8 |
1.8 ± 1.6 |
1.9 ± 1.9 |
-0.9 |
1.1 |
0.917 |
-14.9 |
|
IL-6, pg/mL) |
2.1 ± 0.8 |
2.3 ± 1.0 |
1.8 ± 0.7 |
-0.1 |
0.7 |
0.062 |
-11.4 |
|
Adiponectin, µg/mL |
4.2 ± 2.7 |
4.5 ± 2.5 |
5.8 ± 4.2 |
-2.2 |
-0.3 |
0.024 |
-6.1 (± 16.0) |
|
Average min per day with moderate-vigorous activity |
18.5 ± 16.2 |
20.0 ± 15.6 |
25.6 ± 25.8 |
-14.5 |
0.8 |
0.188 |
-14.0 |
|
Average activity counts/day (÷105) |
2.5 ± 0.1 |
2.3 ± 1.1 |
2.7 ± 1.3 |
-0.7 |
0.2 |
0.179 |
+38.6 |
|
Diabetic knowledge |
11.7 ± 2.6 |
12.2 ± 2.6 |
12.4 ± 2.0 |
-1.8 |
0.4 |
0.395 |
+5.9 |
|
Commitment to a healthy lifestyle |
4.0 ± 1.8 |
3.4 ± 1.9 |
4.0 ± 2.3 |
-1.0 |
1.0 |
0.332 |
0.0 |
Data given as mean±standard deviation 95% confidence intervals are from repeated measures ANOVA (compound symmetry). Percent changes set as the difference between the average values at visit two and baseline divided by the average value at baseline; values in parenthesis are bootstrap standard errors. Scores for ‘diabetic knowledge’ ranged from 0 (lowest knowledge) to 20 (highest knowledge), ‘commitment to a healthy lifestyle, from 0 (lowest determination) to 12 (highest determination), and ‘practicing healthy nutritional choices’ from 0 (poorest nutritional choices) to 30 (best nutritional choices).
BMI: body mass index; BP: blood pressure; ALT: alanine transaminase; HDL: high-density lipoprotein; HbA1c: glycated hemoglobin; LDL: low-density lipoprotein; eGFR: estimated glomerular filtration rate; hsCRP: high-sensitivity C-reactive protein; ESR: erythrocyte sedimentation rate; TNF-α: tumor necrosis factor-alpha; IL-6: interleukin-6.
Table 3: Clinical and laboratory findings in waist ‘non-responders’ and ‘responders’.
|
Waist circumference, cm |
102.1 ± 11.0 |
-1.5 |
1.2 |
- |
+0.1 |
96.9 ± 12.6 |
2.4 |
4.2 |
- |
-3.4 |
|
Weight, kg |
87.4 ± 14.1 |
-2.3 |
0.8 |
0.082 |
+0.9 (± 1.0) |
83.4 ± 14.5 |
1.3 |
4.6 |
0.004 |
-3.5 (± 0.8) |
|
BMI, kg/m2 |
31.2 ± 3.8 |
-0.8 |
0.3 |
0.083 |
+0.9 (± 0.9) |
32.8 ± 4.1 |
0.5 |
1.8 |
0.007 |
-3.5 (± 1.3) |
|
Pulse rate, min |
79.0 ± 17.0 |
-7.6 |
-9.9 |
0.779 |
-1.4 |
86 ± 15 |
-2.1 |
11.6 |
0.374 |
-5.5 |
|
Systolic BP, mmHg |
118.0 ± 10.0 |
-3.9 |
6.8 |
0.583 |
-1.2 |
127.0 ± 14.0 |
-6.3 |
9.5 |
0.809 |
-1.3 |
|
Diastolic BP, mmHg |
73.0 ± 9.0 |
-4.8 |
6.4 |
0.775 |
-1.1 |
81.0 ± 11.0 |
-2.5 |
8.3 |
0.559 |
-3.6 |
|
Fasting blood glucose, mmol/L |
5.9 ± 0.7 |
-0.2 |
0.2 |
0.316 |
-0.2 |
5.4 ± 0.8 |
-0.3 |
0.3 |
0.460 |
-0.7 |
|
HbA1c, % |
5.6 ± 0.3 |
-0.1 |
0.3 |
0.014 |
-1.9 (± 1.2) |
5.6 ± 0.6 |
-0.1 |
0.4 |
0.212 |
-3.3 (± 1.4) |
|
Total cholesterol, mmol/L |
5.1 ± 1.0 |
-0.6 |
0.5 |
0.146 |
+0.9 |
5.0 ± 1.2 |
0.3 |
0.4 |
0.639 |
-0.6 |
|
Triglyceride, mmol/L |
1.6 ± 0.8 |
-0.3 |
0.4 |
0.879 |
-2.1 |
1.1 ± 0.6 |
-0.2 |
0.2 |
0.801 |
-2.2 |
|
HDL, mmol/L |
1.2 ± 0.3 |
-0.2 |
0.0 |
0.177 |
+9.7 |
1.3 ± 0.4 |
-0.2 |
0.0 |
0.322 |
+6.5 |
|
LDL, mmol/L |
3.2 ± 0.8 |
-0.2 |
0.3 |
0.629 |
-1.5 |
3.1 ± 0.9 |
-0.2 |
0.4 |
0.661 |
-3.2 |
|
ALT, unit/L |
31.6 ± 25.1 |
-13.6 |
12.0 |
0.629 |
+2.5 |
31.4 ± 40.4 |
-3.7 |
21.7 |
0.351 |
-28.6 |
|
Urea, mmol/L |
3.1 ± 0.8 |
-0.6 |
0.1 |
0.389 |
+8.5 |
3.3 ± 1.1 |
-0.7 |
0.4 |
0.797 |
+5.3 |
|
Creatinine, mmol/L |
60.1 ± 13.9 |
-5.1 |
2.3 |
0.035 |
+1.4 (± 3.3) |
67.0 ± 19.0 |
0.1 |
8.4 |
0.064 |
-6.4 (± 3.2) |
|
eGFR, mL min-1/1.73 m2 |
118.0 ± 12.1 |
-1.1 |
6.6 |
0.010 |
-2.8 (± 1.9) |
107.7 ± 18.6 |
-7.4 |
0.8 |
0.072 |
+3.1 (± 2.1) |
|
Vitamin D, nmol/L |
30.0 ± 22.4 |
-10.4 |
19.6 |
0.604 |
-0.2 |
44.6 ± 19.0 |
-11.8 |
5.6 |
0.090 |
+7.0 |
|
Average min per day with moderate-vigorous activity |
14.0 ± 9.0 |
-8.3 |
6.9 |
0.538 |
+7.4 (± 16.2) |
23.4 ± 20.2 |
-28.6 |
-1.3 |
0.055 |
+66.1 (± 39.7) |
|
Average activity counts/day (÷105) |
2.3 ± 0.5 |
-0.3 |
0.5 |
0.899 |
-5.0 (± 7.5) |
2.7 ± 1.3 |
-1.5 |
0.1 |
0.070 |
+27.1 (± 15.7) |
|
hsCRP, mg/dL |
3.9 ± 4.0 |
-2.4 |
0.6 |
0.292 |
+22.4 |
8.0 ± 11.1 |
-3.2 |
8.8 |
0.599 |
-34.9 |
|
ESR, mm/hr |
18.8 ± 11.5 |
-1.9 |
5.0 |
0.646 |
-8.2 |
16.9 ± 9.8 |
-1.0 |
6.1 |
0.344 |
-15.2 |
|
TNF-α, pg/mL |
1.7 ± 1.0 |
-1.2 |
0.9 |
0.914 |
+8.2 |
2.5 ± 2.4 |
-1.2 |
2.1 |
0.647 |
-18.8 |
|
IL-6, pg/mL |
1.9 ± 0.6 |
-0.2 |
0.7 |
0.292 |
-11.0 |
2.4 ± 0.9 |
-0.3 |
1.1 |
0.321 |
-17.5 |
|
Adiponectin, µg/mL |
3.6 ± 1.6 |
-0.8 |
0.5 |
0.713 |
+16.3 (± 12.8) |
4.7 ± 3.4 |
-4.0 |
-0.6 |
0.013 |
+56.0 (± 26.2) |
|
Diabetic knowledge |
11.1 ± 2.6 |
-3.2 |
0.1 |
0.094 |
+13.7 (± 8.2) |
12.0 ± 2.6 |
-1.3 |
1.6 |
0.862 |
+1.3 (± 5.3) |
|
Planning to adopt healthy habits |
3.6 ± 2.3 |
-2.3 |
1.0 |
0.383 |
+17.7 |
4.4 ± 1.4 |
-0.2 |
1.9 |
0.247 |
-18.8 |
#‘Responders’ are participants with ≥ 2 cm decrease in waist circumference at six months. One participant refused the waist circumference measurements and was not included in this analysis.
Data presented as mean±standard deviation. 95% confidence intervals are repeated measures ANOVA (compound symmetry). Percent changes were set as the difference between the average values at visit two and baseline divided by the average value at baseline; values in parenthesis are bootstrap standard errors. Scores for ‘diabetic knowledge’ ranged from 0 (lowest knowledge) to 20 (highest knowledge), ‘commitment to a healthy lifestyle, from 0 (lowest determination) to 12 (highest determination), and ‘practising healthy nutritional choices’ from 0 (poorest nutritional choices) to 30 (best nutritional choices).
BMI: body mass index; BP: blood pressure; ALT: alanine transaminase; HDL: high-density lipoprotein; HbA1c: glycated hemoglobin; LDL: low-density lipoprotein; eGFR: estimated glomerular filtration rate; hsCRP: high-sensitivity C-reactive protein; ESR: erythrocyte sedimentation rate; TNF-α: tumor necrosis factor-alpha; IL-6: interleukin-6.
Discussion
At six months, our educational study has shown favorable outcomes in improving waist-circumference, HbA1c, HDL, serum creatinine, eGFR, and adiponectin level. Considering the high prevalence of T2DM in the UAE, the recently published Emirates Diabetes Society Guidelines recommended screening for diabetes in all adults > 30 years old with first-degree relatives with T2DM.13 A comprehensive lifestyle modification program should be offered to individuals at risk of developing T2DM, including weight management, medical nutrition therapy, and exercise. Diabetes prevention correlated most strongly with weight loss with 16.0% risk reduction per kg of weight loss.14 Studies such as the Finnish Diabetes Prevention Study15 and the Diabetes Prevention Program16 have shown that lifestyle changes can achieve weight reduction and slow the progression to diabetes.
By the end of the study period, more than 25.0% of the participants did not firmly accept the core study messages (i.e., positive family history of diabetes is a risk factor, yet a healthier diet and exercise can change the odds). In addition, most of the improved awareness and motivation did not translate into actual changes in eating and activity habits. Firm commitments to a healthy diet and regular exercise were present only in 25.0% and 31.3% of the participants, respectively. These results highlight barriers to endorse significant health changes in our community. These challenges require thorough assessments and management. Nevertheless, our results show the program is acceptable to local people, and its steps are feasible (as most participants demonstrated good compliance with the scheduled visits). In addition, the planned diabetes prevention education was delivered according to the protocol, which proved feasibility.
Failure to control the rising health risks in the region will result in a large population with deleterious medical complications. A recent study from Ras al-Khaimah, UAE showed 24% of school children were obese or extremely obese.4 Most of these children have a family history of diabetes and cardiovascular disease, which further increases their risk of developing these conditions. Therefore, healthy eating and good exercise habits need to be introduced early and enforced for life. This need is especially relevant since health illiteracy is very common in our region.11,12
Waist circumference was used here as a measure of central (abdominal) obesity [Table 2 and Figure 2]. Participants with ≥ 2 cm reduction were labeled as ‘responders’. At the beginning of the study, these individuals had a better lipid profile (although statistically insignificant) and higher vitamin D than non-responders [Table 3]. In addition, they demonstrated improved cardiac (pulse rate and blood pressure), hepatic (ALT), renal (serum creatinine and eGFR), diabetic (fasting blood glucose and HbA1c ), lipid profile (total cholesterol, TG, HDL, and LDL), physical activity (average minutes per day with moderate-vigorous activity and average activity counts per day), adiponectin, inflammatory (hsCRP, erythrocyte sedimentation rate, TNF-α, and IL-6) markers during the study period [Table 3]. This progress is encouraging and needs to be rewarded and endorsed for life. However, trends of our medical practice allow less time for revealing good health information (as larger efforts are spent on electronic documentation). As a result, the public ‘health awareness deficiency’ is being fulfilled by unacceptable advertisements that dominate our perception of proper food and exercise.17 These worldwide problems necessitate national education policies and regulations.
Prevention of diabetes requires adopting healthy habits despite daily dietary challenges.15,18–20 Previous research has shown action planning and maintenance are strongly influenced by perceived self-efficacy, and individuals are more likely to change and maintain their behavior if they believe in their ability to perform a specific action despite having to deal with barriers.21 The perception of good health and adequate physical activity should be driven by trusted, widely-disseminated health information via reliable websites, electronic messages, and mass media. Motivation techniques (e.g., text messaging that disseminates effective health preventive strategies), goal setting, and attractive action planning must be family-based.7 These methods are shown to be somewhat effective in our community [Table 1 and Table 2].
Limitations of this pilot study are its non-randomized observational design, small sample size, unequal gender ratio, potential selection bias, and short study duration. In addition, the probability and potential impacts of unmeasured confounding factors should be considered. Generalizability of the results, thus, requires large-scale randomized control trial studies.
In a clinical setting, healthcare providers exhibit less emphasis (time and effort) on chronic disease prevention. Counseling on obesity, diabetes, and cardiovascular disease usually occurs when patients present morbidities and physicians tend to concentrate on prescribing pharmaceutical agents rather than establishing healthy habits (good diet and exercise). Consequently, and to our knowledge, nutrition and exercise are mostly driven by commercial advertisements. This study suggests that reaching out to persons with overweight and obese, and a positive family history of T2DM is helpful to raise diabetes risk awareness. The DiAlert based structured diabetes prevention education program targeting subjects with a high risk of developing T2DM is promising and may help reduce the risk of DM. In this country, effective preventive strategies require national health policies that deliver deliberate health education to the entire community.
Conclusion
Our study did not observe significant behavior changes but differences between baseline and visit two (six months) were observed in waist-circumference, HbA1c, HDL, serum creatinine, eGFR, and adiponectin level. The rapidly rising rates of obesity, diabetes, and cardiovascular disease in the UAE require urgent implementation of effective national health policies, aiming at enlightening people about the risk of developing these diseases and the measures to prevent and control them.1
Disclosure
The authors declared conflicts of interest. Educational unconditional funding was granted from Eli Lilly & Company to cover the expenses of the study and the frequent consultation and workshops between Virje University Medical Center and the UAE group.
Acknowledgements
The authors are in debt to all participants who endured frequent consultations and blood testing and to Tawam Diabetes Center (Al Ain, UAE) staff for their valued support.
references
- 1. International Diabetes Federation. International diabetes atlas. 2015.
- 2. Annis AM, Caulder MS, Cook ML, Duquette D. Family history, diabetes, and other demographic and risk factors among participants of the National Health and Nutrition Examination Survey 1999-2002. Prev Chronic Dis 2005 Apr;2(2):A19.
- 3. Harding A-H, Griffin SJ, Wareham NJ. Population impact of strategies for identifying groups at high risk of type 2 diabetes. Prev Med 2006;42(5):364-368.
- 4. AlBlooshi A, Shaban S, AlTunaiji M, Fares N, AlShehhi L, AlShehhi H, et al. Increasing obesity rates in school children in United Arab Emirates. Obes Sci Pract 2016 Jun;2(2):196-202.
- 5. Diabetes Prevention Program Research Group, Knowler WC, Fowler SE, Hamman RF, Christophi CA, Hoffman HJ, et al. 10-year follow-up of diabetes incidence and weight loss in the diabetes prevention program outcomes study. Lancet 2009;374(9702):1677–1686.
- 6. Al-Lawati JA. Diabetes mellitus: a local and global public health emergency! Oman Med J 2017;32(3):177.
- 7. Heideman WH, Nierkens V, Stronks K, Middelkoop BJ, Twisk JW, Verhoeff AP, et al. DiAlert: a lifestyle education programme aimed at people with a positive family history of type 2 diabetes and overweight, study protocol of a randomised controlled trial. BMC Public Health 2011 Sep;11(1):751.
- 8. Heideman WH, de Wit M, Middelkoop BJ, Nierkens V, Stronks K, Verhoeff AP, et al. Diabetes risk reduction in overweight first degree relatives of type 2 diabetes patients: effects of a low-intensive lifestyle education program (DiAlert) a randomized controlled trial. Patient Educ Couns 2015 Apr;98(4):476-483.
- 9. van Esch SC, Cornel MC, Geelhoed-Duijvestijn PH, Snoek FJ. Family communication as strategy in diabetes prevention: an observational study in families with Dutch and Surinamese South-Asian ancestry. Patient Educ Couns 2012 Apr;87(1):23-29.
- 10. Heideman WH, Middelkoop BJ, Nierkens V, Stronks K, Verhoeff AP, van Esch SC, et al. Changing the odds. What do we learn from prevention studies targeted at people with a positive family history of type 2 diabetes? Prim Care Diabetes 2011;5(4):215-221.
- 11. Al-Kaabi JM, Al Maskari F, Cragg P, Afandi B, Souid A-K. Illiteracy and diabetic foot complications. Prim Care Diabetes 2015 Dec;9(6):465-472.
- 12. Al-Kaabi JM, Al Maskari F, Zoubeidi T, Abdulle A, Shah SM, Cragg P, et al. Prevalence and determinants of peripheral neuropathy in patients with type 2 diabetes attending a tertiary care center in the United Arab Emirates. J Diabetes Metab 2014;5(346):3.
- 13. Alawadi F, Abusnana S, Afandi B, Aldahmani KM, Alhajeri O, Aljaberi K, et al. Emirates diabetes society consensus guidelines for the management of type 2 diabetes mellitus–2020. Dubai Diabetes and Endocrinology Journal 2020;26(1):1-20.
- 14. Hamman RF, Wing RR, Edelstein SL, Lachin JM, Bray GA, Delahanty L, et al. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care 2006;29(9):2102-2107.
- 15. Tuomilehto J, Lindström J, Eriksson JG, Valle TT, Hämäläinen H, Ilanne-Parikka P, et al; Finnish Diabetes Prevention Study Group. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001 May;344(18):1343-1350.
- 16. Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002 Feb;346(6):393-403.
- 17. Kreuter MW, Lukwago SN, Bucholtz DC, Clark EM, Sanders-Thompson V. Achieving cultural appropriateness in health promotion programs: targeted and tailored approaches. Heal Educ Behav 2003;30(2):133-146.
- 18. Uusitupa MI, Stancáková A, Peltonen M, Eriksson JG, Lindström J, Aunola S, et al. Impact of positive family history and genetic risk variants on the incidence of diabetes: the Finnish diabetes prevention study. Diabetes Care 2011 Feb;34(2):418-423.
- 19. Whitford DL, Al-Sabbagh M. Cultural variations in attitudes towards family risk of diabetes. Diabetes Res Clin Pract 2010 Nov;90(2):173-181.
- 20. Yamaoka K, Tango T. Efficacy of lifestyle education to prevent type 2 diabetes: a meta-analysis of randomized controlled trials. Diabetes Care 2005 Nov;28(11):2780-2786.
- 21. Schwarzer R, Lippke S, Luszczynska A. Mechanisms of health behavior change in persons with chronic illness or disability: the Health Action Process Approach (HAPA). Rehabil Psychol 2011 Aug;56(3):161-170.