Primary hyperaldosteronism (PA) is a common disease with a prevalence of 5–10% in unselected patients with hypertension.1 It is well known that PA leads to increased cardiovascular morbidity and mortality independent of hypertension. The typical manifestations of PA include hypertension, hypokalemia, and metabolic alkalosis.
Nephrocalcinosis refers to diffuse calcification in the renal parenchyma, and the term is more or less synonymous with medullary nephrocalcinosis. While the term ‘nephrocalcinosis’ was first coined in association with primary hyperparathyroidism (PHPT), several conditions have been linked to this condition. The three commonest causes of nephrocalcinosis are PHPT, distal renal tubular acidosis (dRTA), and medullary sponge kidney.2,3 The diagnosis is purely radiological and can be demonstrated on plain radiographs, ultrasound, or computed tomography (CT). The pathophysiology of nephrocalcinosis includes sustained hypercalcemia and hypercalciuria, increased tubular delivery of calcium to the interstitium, and subsequent deposition based on local conditions, including the pH and inhibitory ions.4
PA is not a recognized cause of nephrocalcinosis and/or nephrolithiasis. There are few case reports linking PA with nephrocalcinosis and/or nephrolithiasis.2,5–10 We report three cases where PA was possibly associated with medullary nephrocalcinosis. Postulated theories linking PA with nephrocalcinosis are also discussed.
Table 1: Biochemical parameters of patients.
|
Serum pH |
7.48 |
7.50 |
7.46 |
7.35–7.45 |
|
Serum bicarbonate, meq/L |
29.8 |
27.7 |
28.1 |
18–22 |
|
Serum potassium, meq/L |
1.4 |
2.0 |
2.4 |
3.5–5.5 |
|
Serum sodium, meq/L |
156 |
140 |
148 |
135–145 |
|
Serum calcium, mg/dL |
9.1 |
9.0 |
8.7 |
8.8–10.5 |
|
PAC, ng/dL |
37.3 |
36.4 |
14.9 |
1.7–23.2 |
|
PRA, ng/mL/hr |
0.25 |
0.10 |
0.04 |
0.15–2.33 |
|
ARR, ng/dL per ng/mL/hr |
149.2 |
364.0 |
374.7 |
< 30.0 |
PAC: plasma aldosterone concentration; PRA: plasma renin activity; ARR: aldosterone renin ratio.
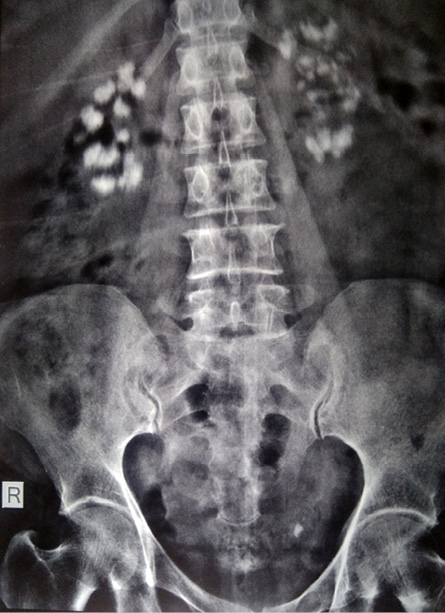
Figure 1: Radiograph of the abdomen showing bilateral nephrocalcinosis.
Case report
Case one
A 35-year-old woman with hypertension for five years was brought to our emergency room with sudden onset loss of consciousness following palpitations without abnormal body movements. On reception, her electrocardiogram showed ventricular tachycardia (VT) along with severe hypokalemia, metabolic alkalosis, and hypernatremia [Table 1]. She was initially treated for VT as per our institutional protocol, along with intravenous potassium replacement. After stabilization, she was evaluated for the cause of hypokalemia. Two similar episodes the previous year were mistaken as seizure and evaluated with magnetic resonance imaging (MRI) of the brain and electroencephalogram (EEG) that were unremarkable. A review of her records revealed hypokalemia and metabolic alkalosis during those episodes, which the treating physician unfortunately overlooked. There was a strong clinical possibility of PA given her poorly controlled hypertension on three antihypertensive agents (telmisartan 40 mg, amlodipine 10 mg, and metoprolol 100 mg), recurrent unprovoked severe hypokalemia leading to VT, and metabolic alkalosis and high serum sodium levels. She was taken off her medication for two weeks and prescribed prazosin sustained-release tablets, verapamil, and oral potassium replacement. After two weeks, a morning blood sample (8:00 AM) was drawn for plasma aldosterone concentration (PAC) and plasma renin activity (PRA). PAC was exceedingly high, and PRA was suppressed [Table 1] with aldosterone renin ratio (ARR) highly suggestive of PA in the given clinical setting.
Contrast-enhanced CT (CECT) of the abdomen with adrenal protocol revealed a right adrenal adenoma measuring 1.3 × 1.2 cm with non-contrast hounsfield units (HU) of 15, absolute percentage washout of 86.7%, and relative percentage washout of 61%. Both kidneys were normal without nephrocalcinosis or renal cysts. Laparoscopic right adrenalectomy was performed, and the resected tissue was reported as adrenal adenoma on histopathological examination. Soon after surgery, the hypokalemia was corrected, and blood pressure declined. Over the following several months, antihypertensive medications were phased out. Following five years of follow-up, she is normokalemic and normotensive without treatment.
Three years after surgery, a radiograph of the abdomen for abdominal pain revealed renal medullary nephrocalcinosis [Figure 1]. A detailed work-up was undertaken for the etiology of medullary nephrocalcinosis. She had normal serum pH (7.39, 7.36), calcium (9.2, 8.9 mg/dL), phosphorus (3.78, 3.73 mg/dL) and normal 24-hour urinary calcium (79.8 mg). We performed ammonium chloride loading test, the results of which showed adequate urinary acidification in response to the acid load. These results ruled out incomplete dRTA as the urine pH level declined below 5.5.
Case two
A 38-year-old woman who had hypertension from the age of 30 with poor control, on three medications, presented to our emergency room with acute pure motor quadriparesis lasting 24 hours. Her investigations revealed metabolic alkalosis with severe hypokalemia [Table 1]. Her quadriparesis resolved promptly within six hours with correction of hypokalemia. Given her poorly controlled early-onset hypertension along with unprovoked severe hypokalemia, PA was suspected. All offending drugs that could affect ARR measurements were stopped for two weeks. After two weeks, a morning sample (8:00 AM) was drawn for PAC and PRA. PAC was exceedingly high, and PRA was suppressed with ARR highly suggestive of PA [Table 1]. CECT adrenals revealed a right adrenal adenoma of size 2.3 × 1.3 cm with unenhanced attenuation of 4 HU. Her CT incidentally revealed bilateral medullary nephrocalcinosis without renal cysts. She underwent laparoscopic right adrenalectomy, followed by a prompt reduction in the requirement for antihypertensive medication and hypokalemia correction. Like case 1, a detailed workup was undertaken for the etiology of medullary nephrocalcinosis and was negative. At one year follow-up, she has well-controlled blood pressure on a single antihypertensive medication without hypokalemia.
Case three
A 28-year-old woman was referred to us for evaluation of resistant hypertension lasting three years. Her medical history revealed intrauterine death attributed to uncontrolled hypertension. Her investigations revealed metabolic alkalosis with severe hypokalemia [Table 1]. A morning blood sample (8:00 AM) was drawn for PAC and PRA. PAC was exceedingly high, and PRA was suppressed with ARR highly suggestive of PA [Table 1]. CECT abdomen revealed a right adrenal adenoma. CECT incidentally revealed bilateral medullary nephrocalcinosis. She underwent laparoscopic right adrenalectomy, with histopathological examination of resected tissue reported as adrenal adenoma. On follow-up for more than six years, she is normokalemic and off antihypertensive agents. Like the first two cases, workup for the etiology of medullary nephrocalcinosis was negative.
In all three cases, the common causes of medullary nephrocalcinosis were excluded by careful clinical history, biochemical evaluation, and radiological findings.
Discussion
Medullary nephrocalcinosis is a type of metastatic calcification that usually arises due to conditions that either increase serum calcium or urinary calcium excretion. The commonest causes of medullary nephrocalcinosis include PHPT, dRTA, and medullary sponge kidney. The uncommon causes include Bartter syndrome, Sarcoidosis, Dent disease, vitamin D toxicity, oculocerebrorenal syndrome, milk alkali syndrome, idiopathic hypercalciuria, Liddle syndrome, Lowe syndrome, primary hyperoxaluria, X-linked hypophosphatemia, Williams syndrome, Wilson’s disease, and prolonged use of diuretics.2
PA is not a recognized cause of nephrocalcinosis and/or nephrolithiasis. There are few case reports linking hyperaldosteronism with nephrocalcinosis and/or nephrolithiasis.2,5–10 The first case of PA associated with nephrocalcinosis was reported by Ogihara et al,5 in a patient who was normokalemic.In 1994, Yang et al,8 reported nephrocalcinosis associated with PA in a 45-year-old woman and implicated chronic hypokalemia as the cause of nephrocalcinosis. In 2015, Mittal et al,2 reported a series of five patients, two with PA and three with secondary hyperaldosteronism associated with nephrocalcinosis. In this series, all five patients had severe chronic hypokalemia. In our series, all three cases had severe chronic hypokalemia.
Various pathogenic mechanisms relating hyperaldosteronism with medullary nephrocalcinosis have been proposed. The earliest proposed mechanism is that chronic hypokalemia secondary to hyperaldosteronism can cause a tubular interstitial injury. This is associated with elevated ammonia genesis, and subsequent renal damage through ammonia-activated alternate complement pathway. The renal cyst formation, interstitial inflammation, and medullary nephrocalcinosis may be related to ammonia-mediated nephropathy.8,11 This has been aptly described as chronic kaliopenic nephropathy. A careful look at all the previously published cases reveals severe chronic hypokalemia in all cases with normokalemia in a single case. We believe this was the likely mechanism in our three cases as well as all three had severe chronic hypokalemia.
Hypercalciuria occurring in hyperaldosteronism is a well-recognized mechanism by which PA can cause nephrocalcinosis. Urinary calcium correlates with sodium excretion; each 100 mEq/dL increment in sodium excretion promotes an increase of 40 mg/dL in calcium excretion.12 Increased urinary calcium excretion in PA could be due to the reduced reabsorption of sodium in aldosterone-insensitive tubular sites.13 Hypercalciuria was the unlikely mechanism in our three cases as we documented normal 24-hour urinary calcium excretion in all cases. Other proposed mechanisms include metabolic alkalosis associated with hypokalemic states, causing decreased calcium phosphate or oxalate solubility in the alkaline urine, thus predisposing to nephrocalcinosis.14 Another mechanism involves PA-induced hypocitraturia.10 It is possible that through multiple ways, PA creates a milieu favorable for nephrocalcinosis
Though we have not proven a direct causal association between PA and nephrocalcinosis in our three cases, we arrived at this possibility after ruling out the common etiologies of nephrocalcinosis.
In our first case, the detection of nephrocalcinosis six months after curative resection of adrenal adenoma casts doubts on the causal association between the two conditions. On the other hand, it may indicate the progression of microscopic to macroscopic nephrocalcinosis.
Conclusion
We conclude and emphasize that medullary nephrocalcinosis could be a manifestation of PA. PA as an etiology of medullary nephrocalcinosis should be sought after the common causes have been excluded, at least in those with hypertension that is difficult to control.
Disclosure
The authors declared no conflicts of interest. Consent was obtained from all three patients.
references
- 1. Funder JW, Carey RM, Mantero F, Murad MH, Reincke M, Shibata H, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an Endocrine Society clinical
- 2. Mittal K, Anandpara K, Dey AK, Sharma R, Thakkar H, Hira P, et al. An association of chronic hyperaldosteronism with medullary nephrocalcinosis. Pol J Radiol 2015 Sep;80:417-424.
- 3. Basak RC, Sharkawi KM, Rahman MM, Swar MM. Distal renal tubular acidosis, hypokalemic paralysis, nephrocalcinosis, primary hypothyroidism, growth retardation, osteomalacia and osteoporosis leading to pathological fracture: a case report. Oman Med J 2011 Jul;26(4):271-274.
- 4. Rumack CM, Wilson SR, Charboneau JW, Levine D. Diagnostic ultrasound: general adult. 4th edition. p. 346-348.
- 5. Ogihara T, Maruyama A, Hata T, Imanaka S, Kumahara Y, Matsumiya K, et al. A case of normoreninemic, normotensive primary aldosteronism associated with essential hypertension and nephrocalcinosis. Clin Exp Hypertens 1981;3(6):1121-1132.
- 6. Yasuda K, Sasaki K, Yamato M, Rakugi H, Isaka Y, Hayashi T. A case of nephrocalcinosis associated with primary aldosteronism. Intern Med 2012;51(6):625-627.
- 7. Kabadi UM. Renal calculi in primary hyperaldosteronism. J Postgrad Med 1995 Jan-Mar;41(1):17-18.
- 8. Yang CW, Kim SY, Kim YS, Koo WS, Choi EJ, Chang YS, et al. Nephrocalcinosis associated with primary aldosteronism. Nephron 1994;68(4):507-508.
- 9. Shey J, Cameron MA, Sakhaee K, Moe OW. Recurrent calcium nephrolithiasis associated with primary aldosteronism. Am J Kidney Dis 2004 Jul;44(1):e7-e12.
- 10. Tantisattamo E, Francis TB. Primary hyperaldosteronism as a risk factor for recurrent nephrolithiasis. Kidney Res Clin Pract 2012 Jun;31:A78.
- 11. Torres VE, Young WF Jr, Offord KP, Hattery RR. Association of hypokalemia, aldosteronism, and renal cysts. N Engl J Med 1990 Feb;322(6):345-351.
- 12. Sakhaee K, Harvey JA, Padalino PK, Whitson P, Pak CY. The potential role of salt abuse on the risk for kidney stone formation. J Urol 1993 Aug;150(2 Pt 1):310-312.
- 13. Rossi E, Perazzoli F, Negro A, Sani C, Davoli S, Dotti C, et al. Acute effects of intravenous sodium chloride load on calcium metabolism and on parathyroid function in patients with primary aldosteronism compared with subjects with essential hypertension. Am J Hypertens 1998 Jan;11(1 Pt 1):8-13.
- 14. Schwedler SB, Gröne EF, Luft FC. Chronic hypokalaemia and nephrocalcinosis. NDT Plus 2009 Aug;2(4):314-317.