Labor pain (LP) is one of the most important causes of concern in pregnant women during vaginal delivery, and pain control is an important maternal consideration.1,2 The management of LP through decreasing maternal anxiety and fear can lead to increased maternal collaboration in the labor progression and reduce the probability of cesarean delivery.3,4 In addition, pain management not only reduces the maternal and neonatal complications secondary to reduced secretion of catecholamines, but also helps improve fetal oxygenation and Apgar scores.5,6 Neuraxial anesthesia, as well as inhalation and injection interventions, are the main methods to control LP.6 More than 40% of women in many centers do not receive neuraxial and local anesthesia due to medical contraindications or self-avoidance secondary to cultural beliefs and inadequate education.7,8 Inhalation interventions have not been taken into consideration by experts due to multiple contraindications, the high frequency of nausea and vomiting, and excessive fatigue after delivery.6
Intravenous opioid analgesics such as morphine and meperidine (pethidine) are among the other pain control methods. The ease of use of intravenous procedures that do not require special conditions and an anesthesiologist has meant that this drug category is a desirable way to reduce LP in maternity hospitals.6 However, studies have shown that the use of opioid analgesics sometimes causes maternal complications such as nausea and vomiting, sleepiness, respiratory depression, and even aspiration.9–11 It is also reported that opioid analgesics may remain in the neonatal bloodstream for about one week due to their ability to cross the placenta and may reduce the neonatal Apgar score due to respiratory distress.9,12,13 Opioid analgesics can cause changes in variability, accelerations, and decelerations through altering the fetal heart rate.14 Therefore, efforts are continuing to search for other intravenous analgesics. Paracetamol (acetaminophen) is the most commonly used analgesic to control pain and fever. Recently, several studies have suggested the use of intravenous acetaminophen to manage LP.2,9,11,15,16 Intravenous acetaminophen through its high plasma concentrations leads to effective pain control by inhibiting the synthesis of prostaglandins in the central nervous system and antagonizing bradykinin.17 Although in the majority of studies acetaminophen infusion controlled pain had comparable efficacy to opioid analgesics, there are few studies assessing maternal and neonatal safety of acetaminophen for LP management, and so it seems necessary to perform further studies in this regard. Therefore, this study aimed to compare the pain score and maternal and neonatal complications following acetaminophen and pethidine injections during vaginal delivery.
Methods
This randomized, double-blind clinical trial was conducted on pregnant women during the first stage of delivery referred to Ghaem and Omolbanin Hospitals in Mashhad, Iran, from March to December 2017.
Before entering the study and after obtaining the required explanations, the patients completed the informed consent form. This research proposal (code No. 941225) was approved by the Ethics Committee of Mashhad University of Medical Sciences (Code of Ethics: IR.MUMS.FM.REC.1395.96). The patients were fully supported by the researchers in the event of any research-related complication, and the patients were free to leave at each stage of the study. This study was also registered in the Iranian Registry of Clinical Trials (IRCT2017100736610N1).
The inclusion criteria were: 1) term pregnancy; 2) singleton pregnancy with cephalic presentation; 3) maternal request for pain relief; 4) no history of underlying or concurrent illnesses including hepatic, renal, cardiovascular, or respiratory diseases, preeclampsia, diabetes, addiction, allergy to acetaminophen, drug abuse, and G6PD deficiency, no need for labor induction, no fetal distress, and no history of cesarean section; 5) non-use of other painkillers during labor; 6) no growth impairment or fetal abnormalities; and 7) active phase of labor. The exclusion criteria included 1) maternal request for withdrawal; 2) taking other analgesics during the study; and 3) the need for induction.
Sampling was done in a purposive manner. Power calculations suggested a sample size of at least 47 patients in each group was necessary based on the mean comparison of the two populations and considering two-sided α of 5% and study power of 80%, according to the results of a previous study.2 Patients were assigned to one of two groups (acetaminophen or pethidine) in a simple random allocation method using a random allocation table. The study was double-blind, meaning the patient and the obstetrician completing the checklist were blind to the groups. The envelope containing the code and the type of drug, which the physician prepared, was given to the nurse responsible for drug infusion.
At baseline, maternal age, gestational age, information related to childbirth records, body mass index (BMI), gestational age based on first-trimester ultrasound, blood pressure, and cervical dilatation were recorded in the checklist. The pain intensity was measured by visual analog scale before and 15, 60, 120, 180, and 240 minutes after injection. A 10-point graded ruler was shown to each patient to record the pain intensity, with a score of 0 indicating no pain and 10 the most severe pain possible. The patient’s presentation of the pain intensity at any time was recorded. The maternal complications that were documented were: nausea, vomiting, dizziness, dry mouth, and respiratory depression. These were assessed during delivery and 15 and 60 minutes after injection. Additionally, other recorded data were the Apgar score at one and five minutes, as well as the need for cardiopulmonary resuscitation and admission to the neonatal intensive care unit (NICU) during the first 24 hours after delivery.
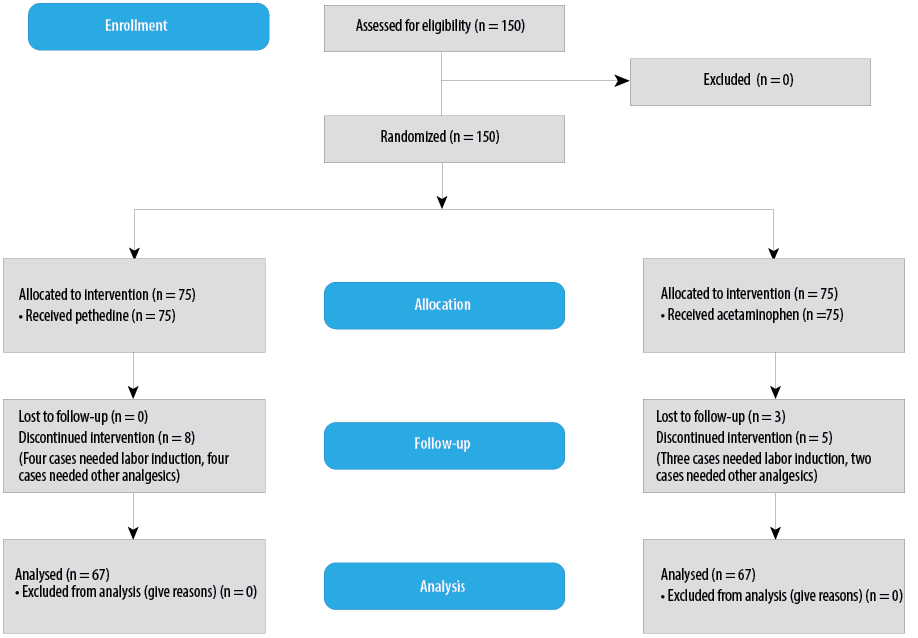
Figure 1: CONSORT flow diagram.
Table 1: Comparison of background variables between pregnant women receiving acetaminophen and pethidine.
|
Age, year* |
26.4 ± 5.6 |
25.2 ± 5.5 |
0.221 |
|
Gravidity** |
|
|
0.008 |
|
1 |
25 (37.3) |
44 (65.7) |
|
|
2 |
18 (26.9) |
12 (17.9) |
|
|
3 |
20 (29.9) |
7 (10.4) |
|
|
4 |
4 (6.0) |
4 (6.0) |
|
|
Parity** |
|
|
0.010 |
|
1 |
27 (40.3) |
45 (67.2) |
|
|
2 |
22 (32.8) |
15 (22.4) |
|
|
3 |
15 (22.4) |
6 (9.0) |
|
|
4 |
3 (4.5) |
1 (1.5) |
|
|
Parity** |
|
|
0.002 |
|
Primiparous |
27 (40.3) |
45 (67.2) |
|
|
Multiparous |
40 (59.7) |
22 (32.8) |
|
|
Type of delivery** |
|
|
|
|
Vaginal |
60 (89.6) |
55 (82.1) |
|
|
Vacuum |
2 (3.0) |
6 (9.0) |
0.315 |
|
Cesarean section |
5 (7.5) |
6 (9.0) |
|
|
Blood pressure before the intervention, mmHg* |
10.8 ± 0.8 |
11.0 ± 0.8 |
0.189 |
|
Dilatation before the intervention, cm* |
5.0 ± 0.9 |
4.9 ± 0.9 |
0.581 |
*Mean±standard deviation.
**Frequency (%).
Table 2: Comparison of labor pain score at pre- and post-intervention between pregnant women receiving acetaminophen and pethidine.
|
Before injection |
1.4 ± 9.2 |
0.7 ± 9.5 |
0.109 |
|
15 minutes |
8.3 ± 1.8 |
8.3 ± 1.6 |
0.880 |
|
60 minutes |
8.2 ± 1.9 |
8.0 ± 2.0 |
0.599 |
|
120 minutes |
8.5 ± 2.0 |
8.6 ± 2.0 |
0.801 |
|
180 minutes |
8.6 ± 2.2 |
9.2 ± 1.4 |
0.251 |
Data were given as mean±standard deviation.
In the acetaminophen group, 1000 mg of acetaminophen (Amp.Tylophen, 1 g, 6.7 mL, Exir Company, Iran) was infused in 100 mL of lactate ringer serum intravenously within 15 minutes. At the same time, 5 cc distilled water was injected in angiocath within two minutes. In the pethidine group, 50 mg of intravenous pethidine (Amp.Petidin 50 mg, 1cc, Exir Company, Iran) was diluted with distilled water to reach a final volume of 5 cc and injected slowly over two minutes intravenously. Simultaneously, 100 cc of lactated ringer was also slowly injected with micro drape for 15 minutes. The initial dose was repeated every four hours if needed according to the patient’s request.
The primary outcome was to compare the pain levels between the two groups after interventions. The secondary outcome was comparing the incidence of maternal and neonatal complications between the two groups after the administration of the drug until discharge from the hospital (at least 24 hours after delivery).
In data analysis, the normal distribution of data was first examined using a one-sample Kolmogorov-Smirnov test with Lilliefors correction. The Pearson’s chi-square test was used to analyze the frequency distribution of qualitative data between the two groups. Moreover, the quantitative variables of the two groups were compared using an independent t-test. The Fisher’s exact test was used in cases where > 20% cells had the expected frequency of < 5 (Cochran). Repeated Measure ANOVA test was used to compare pain intensity changes between the two groups. The statistical software used in this study was IBM SPSS Statistics for Windows (IBM Corp. Released (2010). IBM SPSS Statistics for Windows, Version 19.0, Armonk, NY: IBM Corp.), and the significance level in all tests was < 5%.
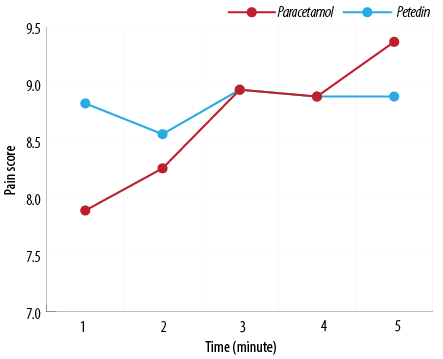
Figure 2: Labor pain score at 15, 60, 120, 180, and 240 minutes after injection between pregnant women receiving acetaminophen and pethidine.
Table 3: Comparison of labor pain score after intervention in the nulliparous and multiparous groups of pregnant women receiving acetaminophen and pethidine.
|
15 minutes |
8.8 ± 1.3 |
8.4 ± 1.5 |
0.313 |
7.9 ± 1.9 |
8.1 ± 1.7 |
0.713 |
|
60 minutes |
8.2 ± 1.7 |
7.9 ± 2.1 |
0.139 |
7.9 ± 2.0 |
8.3 ± 1.8 |
0.468 |
|
120 minutes |
9.1 ± 1.4 |
8.6 ± 2.1 |
0.391 |
7.8 ± 2.3 |
8.5 ± 1.4 |
0.472 |
|
180 minutes |
9.2 ± 1.7 |
9.1 ± 1.5 |
0.790 |
7.6 ± 2.6 |
10.0 ± 0.0 |
0.250 |
Data were given as mean±standard deviation.
Table 4: Comparison of maternal complications during delivery, 15th minute, and the first hour after delivery in pregnant women receiving acetaminophen (n = 67) and pethidine (n = 67).
|
Vomiting |
0 (0.0) |
1 (1.5) |
0.315 |
0 (0.0) |
10 (14.9) |
0.001 |
2 (3.0) |
2 (3.0) |
0.999 |
|
Nausea |
1 (1.5) |
3 (4.5) |
0.310 |
4 (6.0) |
22 (32.8) |
0.001 |
1 (1.5) |
6 (9.0) |
0.052 |
|
Dizziness |
0 (0.0) |
2 (3.0) |
0.154 |
2 (3.0) |
23 (34.3) |
0.001 |
2 (3.0) |
6 (9.0) |
0.145 |
|
Dyspnea |
0 (0.0) |
0 (0.0) |
0.001 |
0 (0.0) |
1 (1.5) |
0.315 |
0 (0.0) |
2 (3.0) |
0.154 |
|
Blurring vision |
0 (0.0) |
0 (0.0) |
0.001 |
0 (0.0) |
3 (4.5) |
0.080 |
0 (0.0) |
0 (0.0) |
- |
Data were given as n(%).
Table 5: Comparison of neonatal complications within the first 24 hours after delivery in the groups receiving acetaminophen and pethidine.
|
Admitted to NICU |
3 (4.5) |
8 (11.9) |
0.116 |
|
Cardiopulmonary resuscitation |
2 (3.0) |
8 (11.9) |
0.049 |
|
One minute Apgar score ≤ 7 |
2 (3.0) |
11 (16.4) |
0.009 |
Data were given as n(%).
NICU: neonatal intensive care unit.
Results
This study was performed on 134 patients with a mean age of 25.8±5.6 years in both groups (acetaminophen, n = 67 and pethidine, n = 67) [Figure 1]. The two groups did not differ significantly in terms of age, labor history, delivery method, BMI, gestational age based on ultrasound, blood pressure, cervical dilatation, and pain score before the intervention, and there was no significant difference in the interval between analgesic infusion and delivery [Table 1]. In addition, there were no abortions or stillbirths in either group.
Point-to-point pain scoring before and 15, 60, 120, 180, and 240 minutes after injection showed that the two groups had no significant difference [Table 2]. The significance level of Pillai’s trace, Wilks’ lambda, Hotelling’s trace, and Roy’s largest root tests was 0.031. The results showed a significant difference between the pain score and the five time points. However, the trend of pain score changes between the two groups did not differ significantly due to the significance level of the tests performed at 0.105 [Figure 2]. Also, there was no significant difference in the mean pain score of delivery in the nulliparous and multiparous groups of pregnant women receiving acetaminophen and pethidine [Table 3]. None of the two groups received additional doses of analgesics to control pain.
The incidence rate of maternal complications during the first hour after injection was significantly different between the two groups, and 15 minutes after the injection, vomiting, nausea, and dizziness were significantly higher in the pethidine group [Table 4]. Mean one and five minutes Apgar score were significantly higher in the acetaminophen group than in the pethidine group (first minute: 8.8±0.6 vs. 7.5±0.9; p = 0.033 and fifth minute: 9.9±0.5 vs. 8.7±0.5; p = 0.004). Also, the number of newborns admitted to the NICU, needed cardiopulmonary resuscitation, and with Apgar scores ≤ 7 were higher in the pethidine group [Table 5].
Discussion
Despite the absence of clinically and statistically significant differences between the two pain management methods, intravenous acetaminophen seems to have fewer complications than pethidine in terms of maternal complications, especially during the first 15 minutes after injection and for neonatal complications, especially in the Apgar score.
The primary finding of our study investigated the effectiveness of acetaminophen on LP management, which has already been confirmed in other studies.2,9,11,15,16 However, our study also included multigravida women, had a larger sample size, used more advanced statistical methods, and examined a wider range of maternal and neonatal complications making our findings more remarkable. On the other hand, only one clinical trial has been conducted on the efficacy of acetaminophen with pethidine in controlling LP in Iranian primigravida women in a small population sample.18 The effectiveness of an analgesic drug may be associated with racial variations with different influences, so we recommend another study in the Iranian population to increase the generalizability of the results.19 In our study, in addition to point-to-point comparison of the pain intensity at follow-up times of 15, 60, 120, 180, and 240 minutes after injection, pain score changes were also compared between the two therapeutic methods that no statistically significant difference was observed between the two groups. Interestingly, there was no significant change in the pain intensity in the acetaminophen group during the follow-up period, so that the pain score varied between 8.3 and 9.3 in most of the time intervals, while the pain intensity of the pethidine group was in the larger range, between 8.0 and 9.6, and was associated with more changes.
It seems that no sudden changes in pain intensity is a benefit of acetaminophen. In other words, although the reduction in primary pain was higher in the pethidine group, it decreased quickly. In contrast, in the case of acetaminophen, the reduction trend in primary pain was less, but the effect of pain relief was more stable. There was at least equal effectiveness of acetaminophen compared to an opioid compound in controlling pain score in our study, and this has also been reported in other studies.2,13,18 However, few studies have compared acetaminophen with pethidine in the control of LP;2,13,18 and in the majority of studies, the effects of acetaminophen have been evaluated in comparison with morphine or tramadol.11,20–22 Among these studies, Abdollahi et al,18 was the most similar in terms of design and intervention type. In this study, the efficacy of 50 mg of pethidine was compared with 1000 mg acetaminophen.18 The results indicated that pethidine was not superior to acetaminophen in controlling acute pain after injection, consistent with our findings. However, this study compared a single degree of pain score after injection between the two groups, while our study compared the pain intensity between the two groups at five time points after the intervention. In addition to point-to-point comparison, the course of the pain changes was also compared. Another advantage of the current study was that we compared specifically the neonatal and the maternal complications between the two groups, while Abdollahi et al, studied the maternal complications individually, and the Apgar score was not compared between the two groups.18 Similarly, Elbohoty et al conducted a study to compare injectable acetaminophen and pethidine in reducing LP, which, as in our study, showed a similar decrease in pain in the two groups.2
Pain intensity was compared between the multigravida and primigravida subgroups. The results showed no difference in the effect of gravidity on pain intensity between the two groups.
The study of Aimakhu et al,20 with a relatively long-term follow-up, showed that intravenous acetaminophen could have long-term pain control for at least four hours following vaginal delivery; this finding is similar to the results of our study.
The maternal and neonatal complications were our secondary outcome. The first significant issue among our findings was that all maternal complications in the pethidine group at three injection times (before, 15 minutes, and the first hour after injection) had equivalent or higher prevalence than acetaminophen. The only exception in this regard was the occurrence of dry mouth in the acetaminophen group during labor, while this complication was not observed in the pethidine group; however, this difference was not statistically significant clinically. In other studies, like ours, a lower incidence of vomiting was found in the acetaminophen group compared to opioids.9,20,22 However, the interesting point is that our study investigated a greater range of complications than other studies. Unlike other studies that reported complications only during follow-up, our study reported the complications during labor, and 15 minutes and 24 hours after childbirth. It seems that our study findings, for the first time, have looked at a more comprehensive view of maternal complications as an important outcome. In some previous studies, complications including nausea, vomiting, orthostatic hypotension, and respiratory distress have a lower incidence in the acetaminophen group than the opioid analgesics,9,10 some of which were confirmed in our study. Our findings showed that blurring vision and dry mouth are other possible complications that are less common in the acetaminophen group. Another point is that, > 30% of women experienced nausea and dizziness in the pethidine group, but the frequency of these two complications in the acetaminophen group was < 6% in the same period, which is clinically very important. Another interesting point is that vomiting did not occur in the first minute after delivery in any of the patients in the acetaminophen group, while this unpleasant event occurred simultaneously in about 15% of patients in the pethidine group. Therefore, it seems that acetaminophen may be safer than opioid analgesics in terms of maternal complications.
Among the neonatal complications, our evidence was also in favor of the higher safety of acetaminophen, so that quantitative and qualitative analyses showed that the Apgar score was significantly higher in the acetaminophen group one and five minutes after delivery compared to pethidine. However, few studies examined quantitatively and qualitatively the Apgar score between the groups of acetaminophen and opioid analgesics, whose findings also showed higher safety of acetaminophen than opioid analgesics or the absence of the difference between the two groups.18,20,22 Mohan et al,22 compared acetaminophen with tramadol and found no differences in the Apgar score at one and five minutes. In our study, the higher frequency of infants admitted to NICU in the pethidine group had no statistically significant difference with the acetaminophen group, but since the studied variable is a death-related outcome, we believe that the clinical differences are very important. Aimakhu et al,20 also concurred with our study and showed that the frequency of infants admitted to the NICU was lower in the acetaminophen group than in the tramadol group (5.6% vs. 0%, p = 0.12). Acetaminophen seems to have fewer neonatal complications than opioid analgesics, although more studies are needed to address the generalizability of these findings, similar to the maternal complications.
There were several limitations in our study. The cervical position at the onset of labor and the size and position of the fetal head are other factors affecting the severity and duration of LP that in our study were not specifically compared between the two groups, but according to random allocation, these factors do not seem to be as the important confounding factors in our outcomes. Another limitation of our study was the failure to compare the state of fetal heart rate as an important factor in controlling the side effects of the drug, and so we were unable to directly investigate the effect of acetaminophen and pethidine on fetal heart rate pattern. Another limitation was the lack of maternal satisfaction evaluation in the two groups. The strength of the current study is the large sample size in primigravida and multigravida women compared with some other studies. In addition, the longer follow-up seems to be one of the advantages of our study design over other studies. Considering the very low side effects observed with acetaminophen, we suggest that future studies should compare the different doses of acetaminophen to achieve the highest level of pain control and maternal satisfaction along with the least maternal and neonatal complications.
Conclusion
According to the results obtained from the present study, acetaminophen appears to be equally effective as pethidine in controlling pain due to vaginal delivery in nulliparous and multiparous women. However, the prevalence of maternal complications, especially nausea, vomiting, and dizziness, was significantly lower in the acetaminophen group than in the pethidine group. In addition, based on the one and five minutes Apgar scores, as well as the need for cardiopulmonary resuscitation in newborn infants, acetaminophen seems to be a safer drug in terms of neonatal complications.
Disclosure
The authors declared no conflicts of interest.
Acknowledgements
The present study has been adopted from the specialty thesis written by Dr. Mozhgan Soltani in obstetrics and gynecology at Mashhad University of Medical Sciences (code: T5008). This study was conducted with the financial and spiritual supports of the Deputy of Research at Mashhad University of Medical Sciences within a proposal approved by Code 941225.
references
- 1. Khadem N, Zirak N, Soltani G, Sahebdelfar N, Sepehri Shamloo A, Ebrahimzadeh S. Comparison of epidural versus entonox for labor analgesia in Nulliparous women. Journal of Surgery and Trauma 2013;1(1):1-5.
- 2. Elbohoty AE, Abd-Elrazek H, Abd-El-Gawad M, Salama F, El-Shorbagy M, Abd-El-Maeboud KH. Intravenous infusion of paracetamol versus intravenous pethidine as an intrapartum analgesic in the first stage of labor. Int J Gynaecol Obstet 2012 Jul;118(1):7-10.
- 3. Alehagen S, Wijma B, Lundberg U, Wijma K. Fear, pain and stress hormones during childbirth. J Psychosom Obstet Gynaecol 2005 Sep;26(3):153-165.
- 4. Shalaby HS, Hemada HM, Faris MA, Ali NH. Efficacy of intravenous tenoxicam as an analgesic during the first stage of labor: a randomized controlled trial. Egyptian Journal of Hospital Medicine 2018;70(4):601-609.
- 5. Alehagen S, Wijma K, Lundberg U, Melin B, Wijma B. Catecholamine and cortisol reaction to childbirth. Int J Behav Med 2001;8(1):50-65.
- 6. Hensley JG, Collins MR, Leezer CL. Pain management in obstetrics. Crit Care Nurs Clin North Am 2017 Dec;29(4):471-485.
- 7. Osterman MJ, Martin JA. Epidural and spinal anesthesia use during labor: 27-state reporting area, 2008. Natl Vital Stat Rep 2011 Apr;59(5):1-13, 16.
- 8. Orejuela FJ, Garcia T, Green C, Kilpatrick C, Guzman S, Blackwell S. Exploring factors influencing patient request for epidural analgesia on admission to labor and delivery in a predominantly Latino population. J Immigr Minor Health 2012 Apr;14(2):287-291.
- 9. Ankumah NE, Tsao M, Hutchinson M, Pedroza C, Mehta J, Sibai BM, et al. Intravenous acetaminophen versus morphine for analgesia in labor: a randomized trial. Am J Perinatol 2017 Jan;34(1):38-43.
- 10. Jain S, Arya VK, Gopalan S, Jain V. Analgesic efficacy of intramuscular opioids versus epidural analgesia in labor. Int J Gynaecol Obstet 2003 Oct;83(1):19-27.
- 11. Kaur Makkar J, Jain K, Bhatia N, Jain V, Mal Mithrawal S. Comparison of analgesic efficacy of paracetamol and tramadol for pain relief in active labor. J Clin Anesth 2015 Mar;27(2):159-163.
- 12. Kim K, Johnson JA, Derendorf H. Differences in drug pharmacokinetics between East Asians and Caucasians and the role of genetic polymorphisms. J Clin Pharmacol 2004 Oct;44(10):1083-1105.
- 13. Olofsson C, Ekblom A, Ekman-Ordeberg G, Hjelm A, Irestedt L. Lack of analgesic effect of systemically administered morphine or pethidine on labour pain. Br J Obstet Gynaecol 1996 Oct;103(10):968-972.
- 14. Sekhavat L, Behdad S. The effects of meperidine analgesia during labor on fetal heart rate. Int J Biomed Sci 2009 Mar;5(1):59-62.
- 15. Abd-El-Maeboud KH, Elbohoty AE, Mohammed WE, Elgamel HM, Ali WA. Intravenous infusion of paracetamol for intrapartum analgesia. J Obstet Gynaecol Res 2014 Nov;40(11):2152-2157.
- 16. Towers CV, Shelton S, van Nes J, Gregory E, Liske E, Smalley A, et al. Preoperative cesarean delivery intravenous acetaminophen treatment for postoperative pain control: a randomized double-blinded placebo control trial. Am J Obstet Gynecol 2018 Mar;218(3):353.e1-353.e4.
- 17. Graham GG, Scott KF. Mechanism of action of paracetamol. Am J Ther 2005 Jan-Feb;12(1):46-55.
- 18. Abdollahi MH, Mojibian M, Pishgahi A, Mallah F, Dareshiri S, Mohammadi S, et al. Intravenous paracetamol versus intramuscular pethidine in relief of labour pain in primigravid women. Niger Med J 2014 Jan;55(1):54-57.
- 19. Anderson KO, Green CR, Payne R. Racial and ethnic disparities in pain: causes and consequences of unequal care. J Pain 2009 Dec;10(12):1187-1204.
- 20. Aimakhu C, Saanu O, Olayemi O. Pain relief in labor: a randomized controlled trial comparing intramuscular tramadol with intramuscular paracetamol at the University College Hospital, Ibadan, Nigeria. Trop J Obstet Gynaecol 2017;34(2):91-98.
- 21. Lallar M, Anam HU, Nandal R, Singh SP, Katyal S. Intravenous paracetamol infusion versus intramuscular tramadol as an intrapartum labor analgesic. J Obstet Gynaecol India 2015 Feb;65(1):17-22.
- 22. Mohan H, Ramappa R, Sandesh M, Akash B. Intravenous paracetamol infusion versus intramuscular tramadol as an intrapartum labor analgesic. Int J Reprod Contracept Obstet Gynecol 2017;4(6):1726-1729.