Fibromuscular dysplasia (FMD) is a common but underdiagnosed clinical entity with an estimated prevalence of around 5%.1 FMD classically involves the renal arteries; however, other vascular territories, including the carotid and coronary arteries, can be affected. Women, particularly young women, are disproportionally affected by this condition. One of the most serious potential complications of FMD is spontaneous coronary artery dissection, leading to myocardial ischemia, infarction, and systolic dysfunction. This report presents a 39-year-old female who rapidly deteriorated into cardiogenic shock secondary to spontaneous coronary dissection from underlying FMD with involvement of the coronary and carotid arteries.
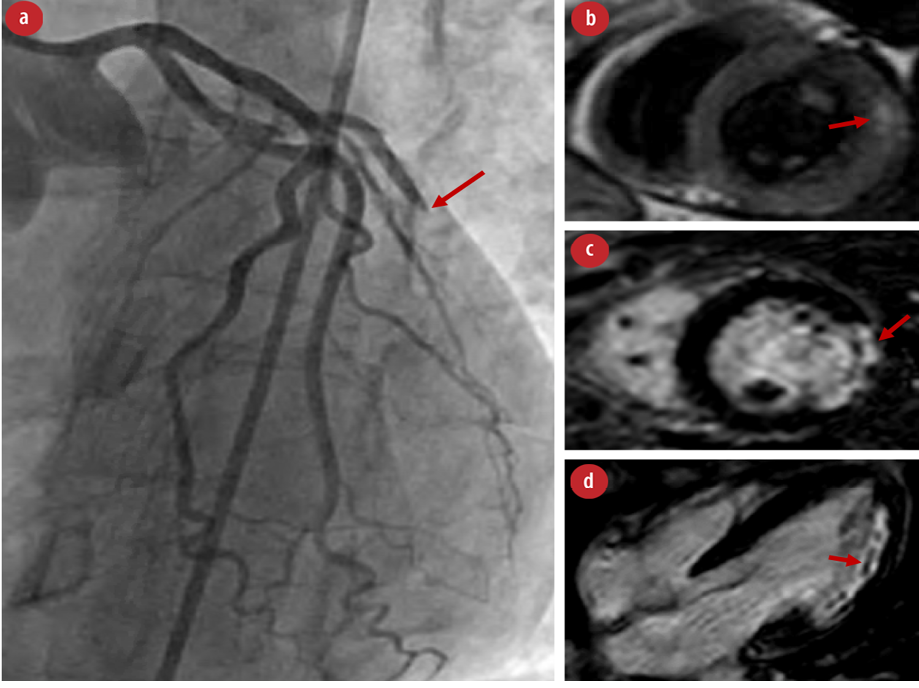
Figure 1: (a) Left heart catheterization with the arrow pointing to ‘acute’ complete occlusion of the obtuse marginal artery. (b) Cardiac MRI with T1-weighted imaging showing myocardial edema involving the basal to mid anterolateral segments corresponding to acute cardiac injury/inflammation. (c) Cardiac MRI showing evidence of ‘near’ transmural infarction of the basal to mid-lateral wall with evidence of late gadolinium enhancement in the short axis and (d) microvascular obstruction as seen in four chambers view.
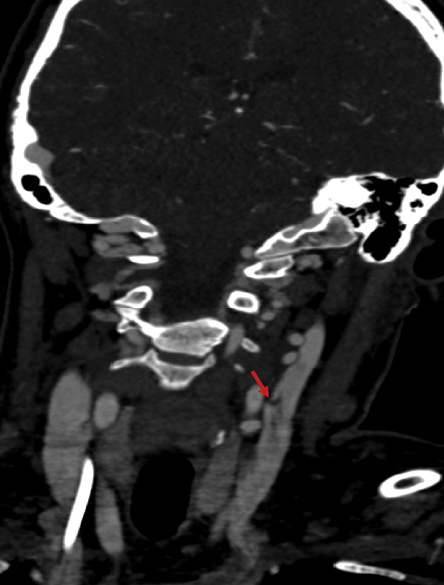
Figure 2: Angiogram of the neck showing dissection of the left cervical internal carotid artery without any evidence of contrast extravasation.
Case report
Our patient was a 39-year-old female with a history of hypertension and preeclampsia who presented to an outpatient visit with sudden onset, severe, substernal chest pain. Other associated symptoms at the time of presentation were diaphoresis, dizziness, and nausea. An electrocardiogram was performed and demonstrated normal sinus rhythm with ST depression V3-V4 and ST elevation in V5-V6. The patient was subsequently transferred to the emergency department for further evaluation. Minutes later, she became unresponsive and was found to be in pulseless ventricular fibrillation. Advanced cardiovascular life support was initiated providing two doses of epinephrine along with one dose of intravenous amiodarone. After three attempts of defibrillation, we achieved successful return of circulation. Bedside echocardiogram demonstrated lateral akinesis with an estimated left ventricular ejection fraction of 10–15%. She was urgently transferred to the cardiac catheterization laboratory, where she had a recurrence of ventricular fibrillation requiring one successful defibrillation attempt. Her cardiac catheterization [Figure 1] demonstrated 100% stenosis of a large first obtuse marginal artery consistent with spontaneous dissection. Initially, an attempt at percutaneous coronary intervention was made. However, given the nature of the dissection, the patient was managed medically. Following catheterization, she continued to decompensate into cardiogenic shock with elevated lactate and hypotension requiring epinephrine continuous infusion and venoarterial extracorporeal membrane oxygenation (VA-ECMO) with 2.5 L/min of support. An echocardiogram performed while on VA-ECMO revealed a left ventricular ejection fraction of 45–50% with severe hypokinesis of the mid to distal inferolateral, mid anterolateral, and the mid anterior wall. She improved clinically throughout the rest of her stay. She was successfully weaned off VA-ECMO on the third hospitalization day.
After stabilization, she underwent further evaluation for underlying vascular pathology. Computed tomography (CT) angiograms of the chest, abdomen, and pelvis were unremarkable, notably with normal renal arteries. CT angiograms of the head and neck revealed bilateral abnormalities of the cervical internal carotid arteries consistent with FMD. A small dissection of the right internal carotid artery and dissection/aneurysm formation of the left cervical internal carotid artery were also noted [Figure 2]. Additionally, high-grade stenosis of the right A3 segment, a subacute posterior cerebellar infarct, and chronic right lacunar infarct were identified. Evaluation by the neurology team revealed no corresponding neurological deficits. On day five, she underwent a cardiac magnetic resonance imaging (MRI), which showed a transmural ‘5-segment’ infarction involving the basal anterolateral, mid-lateral, apical inferior, and apical lateral walls with evidence of tissue edema and microvascular obstruction indicating an acute cardiac injury in the context of edema and inflammation [Figure 1]. This was felt to be consistent with findings of the previous coronary catheterization. The cardiac MRI also showed recovery of the left ventricular systolic function with an ejection fraction of 54%.
Genetic testing via microarray to screen for an underlying connective tissue disease was negative for any pathogenic deletions or duplications. Additional workup included antineutrophil cytoplasmic antibodies (c-ANCA, p-ANCA), antineutrophil antibodies, erythrocyte sedimentation rate, and complement C3/C4, which were all within normal limits or negative. The patient improved clinically, and on day 10, she was discharged on aspirin, metoprolol tartrate, and losartan.
Discussion
We present a case of FMD in a young woman presenting with widespread vascular involvement and a clinical tetrad of spontaneous coronary artery dissection, cardiogenic shock requiring mechanical support, bilateral internal carotid dissections, and subacute cerebellar infarct.
Previously, several cases reports of FMD presenting with spontaneous coronary artery dissection requiring mechanical support and/or cardiac transplantation have been published; however, these reports had less widespread involvement of other vascular territories.2 Previously published work demonstrated that in a cohort of 50 cases of spontaneous coronary artery dissection, 43 patients had FMD involving at least one non-coronary territory. However, the cerebrovascular circulation is the least likely to be involved.3 Interestingly, despite our patient’s extensive and bilateral FMD, she had no renal and iliac involvement, which has been described extensively in this disease state.
Spontaneous coronary artery dissection is a relatively rare but likely underreported disease that has been implicated in approximately 2% of all acute coronary syndrome cases that undergo cardiac catheterization. Spontaneous coronary artery dissection is disproportionately common in young women and might be the underlying cause for the majority of female patients presenting with acute coronary syndrome under the age of 60.4,5 FMD is also strongly associated with the majority of spontaneous coronary artery dissection cases, but the overall prevalence of FMD is low, with some estimates for the prevalence of renal involvement of 0.4%.6 The female predominance of FMD has been well documented. However, there also appears to be an interesting relationship between FMD, spontaneous coronary artery dissection, and recent pregnancy, which was documented in a previous report and the case we present here.2
The management of FMD presenting with spontaneous coronary artery dissection is not well defined. Several case reports, including our case, described patients with spontaneous coronary artery dissection responding successfully to mechanical circulatory support when the dissection is complicated by cardiogenic shock.2,7–9 Furthermore, acute revascularization strategies (especially in unstable patients) and long-term medical management remains undefined. Previous studies have indicated no clear improvement in outcomes following target vessel revascularization versus conservative therapy, with high percutaneous coronary intervention procedural failure rates.10 Likewise, the long-term angiographic and clinical outcomes appear to be favorable with medical therapy alone.11 Pharmacological therapy of spontaneous coronary artery dissection centers on single or dual antiplatelet therapy with the
addition of beta-adrenergic blockers. When spontaneous coronary artery dissection is complicated by heart failure, guideline-directed therapy for heart failure with reduced ejection fraction is indicated.4 However, medical therapy for heart failure with reduced ejection fraction is also recommended in FMD independent of heart failure.4
Conclusion
We present a rare and interesting case of a 39-year-old female with spontaneous coronary artery dissection complicated by cardiogenic shock secondary to previously undiagnosed FMD with the carotid arteries involvement. This case highlights the possible extent of FMD and the potentially devastating complications of this disease. Furthermore, this case further outlines the role of mechanical circulatory support in cardiogenic shock cases secondary to spontaneous coronary artery dissection and the long-term management of affected patients. We recommend further investigations into the acute and long-term management of FMD and spontaneous coronary dissection.
Disclosure
The authors declared no conflicts of interest. The patient gave verbal and written consent for the use of her data in this report.
references
- 1. Shivapour DM, Erwin P, Kim ESh. Epidemiology of fibromuscular dysplasia: a review of the literature. Vasc Med 2016 Aug;21(4):376-381.
- 2. Alonso-Fernández-Gatta M, Uribarri A, Diego-Nieto A, Sánchez PL. Progressive spontaneous coronary artery dissection secondary to fibromuscular dysplasia requiring mechanical circulatory support. J Cardiol Cases 2017 Sep;16(6):216-218.
- 3. Saw J, Ricci D, Starovoytov A, Fox R, Buller CE. Spontaneous coronary artery dissection: prevalence of predisposing conditions including fibromuscular dysplasia in a tertiary center cohort. JACC Cardiovasc Interv 2013 Jan;6(1):44-52.
- 4. Hayes SN, Kim ES, Saw J, Adlam D, Arslanian-Engoren C, Economy KE, et al; American Heart Association Council on Peripheral Vascular Disease; Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Genomic and Precision Medicine; and Stroke Council. Spontaneous coronary artery dissection: current state of the science: a scientific statement from the American heart association. Circulation 2018 May;137(19):e523-e557.
- 5. Rashid HN, Wong DT, Wijesekera H, Gutman SJ, Shanmugam VB, Gulati R, et al. Incidence and characterisation of spontaneous coronary artery dissection as a cause of acute coronary syndrome–a single-centre Australian experience. Int J Cardiol 2016 Jan;202:336-338.
- 6. Varennes L, Tahon F, Kastler A, Grand S, Thony F, Baguet JP, et al. Fibromuscular dysplasia: what the radiologist should know: a pictorial review. Insights Imaging 2015 Jun;6(3):295-307.
- 7. Padukone A, Sayeed AK, Marczin N, García Sáez D, Zych B, Mohite PN, et al. Salvage myocardial revascularisation in spontaneous left main coronary artery dissection with cardiogenic shock - the role of mechanical circulatory support. Perfusion 2017 Mar;32(2):171-173.
- 8. Evans R. Post-partum spontaneous coronary artery dissection and the use of veno-arterial extra-corporeal membrane oxygenation. Nurs Crit Care 2014 Nov;19(6):304-309.
- 9. Knapp KE, Weis RA, Cubillo EI, Chapital AB, Ramakrishna H. Spontaneous, postpartum coronary artery dissection and cardiogenic shock with extracorporeal membrane oxygenation assisted recovery in a 30-year-old patient. Case Rep Cardiol 2016;2016:1048708.
- 10. Tweet MS, Eleid MF, Best PJ, Lennon RJ, Lerman A, Rihal CS, et al. Spontaneous coronary artery dissection: revascularization versus conservative therapy. Circ Cardiovasc Interv 2014 Dec;7(6):777-786.
- 11. Rogowski S, Maeder MT, Weilenmann D, Haager PK, Ammann P, Rohner F, et al. Spontaneous coronary artery dissection: angiographic follow-up and long-term clinical outcome in a predominantly medically treated population. Catheter Cardiovasc Interv 2017 Jan;89(1):59-68.