The complex spongy exterior of vegetables often eases the attachment and survival of pathogens such as enteric viruses, bacteria, and parasites, increasing the likelihood of foodborne infections following the consumption of raw and/or softly cooked vegetables.1,2 Contamination of vegetables often occurs during production, collection, transport, preparation, and/or during processing.3,4 The primary sources of contamination are soil, sewage, human feces, animal manure, and water (irrigation and cleaning). Similarly, both domestic and wild animals contribute directly and significantly to the contamination of vegetables.5 Furthermore, contamination may conceivably occur when the vegetables are dowsed and sprinkled with contaminated water.3,6 Numerous reports have recently emerged from various regions of the world linking an increased number of foodborne illnesses to the consumption of raw vegetables from both developed and developing countries due to poor hygiene and inadequate personal cleanliness.1,4,6–13
With the increased exigency for ready-to-eat foods, particularly those containing raw fresh vegetables, there is massive unease regarding the safety of these products and the threat they may pose to the public in the presence of unhygienic practices and improper management. To the best of our knowledge, there is no published data on the parasitological contamination of fresh vegetables produce in farms and markets in the UAE. Therefore, this study aimed to assess the degree of parasitic contaminations on selected vegetables.
Methods
This study was carried out between February 2017 and January 2018. A total of 218 fresh vegetable samples, including 14 different types frequently consumed in the UAE, were randomly collected from different farms and local supermarkets. Samples collected included: fennel (n = 6), green pepper (n = 6), chard (n = 5), rocket (n = 9), watercress (n = 26), lettuce (n = 20), spring onion (n = 35), tomato (n = 18), radish (n = 17), broccoli (n = 10), parsley (n = 16), mint (n = 20), carrots (n = 14), and cucumber (n = 16). All samples were collected in sterile, labeled polythene bags and transported immediately to the parasitology laboratory at the University of Sharjah for parasitic examination. The information on the label included the sample type and date of collection. All samples were washed by vigorous shaking with 10% formal saline (150 mL) for detaching the parasitic stages (ova, larvae, cysts, and oocysts) of helminths and protozoa parasites presumed to be associated with vegetable contamination. The washing solution was left for about 10 minutes, followed by transfer of 150 mL of the washing solution into three centrifuge tubes (50 mL each). For concentrating the parasitic stages, the three tubes were centrifuged at 3000 rpm for 5 minutes.14 After centrifugation, the supernatant was carefully siphoned off without shaking. The sediment was then agitated gently by hand to redistribute the parasitic stages, collected in one tube, and examined for parasites under a light microscope using 10 × and 40 × objective lenses. For the unstained smear, two slides were prepared from each sample to increase the chance of parasite detection. Data analysis was done using (SPSS Statistics IBM Corp. Released 2012. IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM Corp.). We used the chi-square test to determine the association (if any) between the occurrence of parasites in fresh vegetables and confirm the significance between the parasite detected and the vegetable type. A p-value < 0.050 was considered significant.
Table 1: Distribution of intestinal parasitic contamination in different fresh vegetables.
|
Fennel |
6 |
|
0 (0.0) |
|
Green pepper |
6 |
|
0 (0.0) |
|
Chard |
5 |
|
1 (20.0) |
|
Rocket |
9 |
|
0 (0.0) |
|
Watercress |
26 |
|
4 (15.4) |
|
Lettuce |
20 |
|
3 (15.0) |
|
Spring onions |
35 |
|
8 (22.9) |
|
Tomato |
18 |
|
1 (5.6) |
|
Radish |
17 |
|
5 (29.4) |
|
Broccoli |
10 |
|
3 (30.0) |
|
Parsley |
16 |
|
2 (12.5) |
|
Mint |
20 |
|
4 (20.0) |
|
Carrot |
14 |
|
0 (0.0) |
|
Cucumber |
16 |
|
2 (12.5) |
Results
A total of 218 fresh vegetable samples were examined for the presence of parasitic contamination. Protozoan cysts and helminths eggs were detected in 15.1% (33/218) in 10 out of 14 investigated vegetable samples [Table 1]. The most detected parasites were Entamoeba complex (E. histolytica/E. dispar/E. moshkovskii) (30.3%), Entamoeba coli (18.2%), Trichuristrich trichiura and Strongyloides stercoralis (12.1% each), Ascaris lumbricoides egg (9.1%), Endolimax nana cyst and Enterobius vermicularis egg (6.1% each), and Giardia lamblia and Hymenolepis nana (3.0% each) as shown in Table 2. The most contaminated vegetables in descending order were broccoli (30.0%), radish (29.4%), spring onions (22.9%), and mint and chard (20.0% each). No parasites were detected in fennel, green pepper, rocket, and carrots (0.0%) [Table 1]. Except for two vegetable types, all examined vegetable samples showed multiple parasitic contaminations. The only two vegetables that showed mono-parasite contamination were chard and tomato [Table 2, Figure 1].
Chi-square analysis revealed no significant association between the vegetable type and the parasite occurrence in this study (p > 0.050). Moreover, parasite incidence was independent of the vegetable type (p > 0.050).
Table 2: Distribution of intestinal parasites in relation to the type of fresh vegetables collected from various farms and markets in the UAE.
|
Entamoeba coli cyst |
0 |
1 |
0 |
1 |
0 |
1 |
0 |
0 |
2 |
0 |
1 |
6 (18.2) |
|
Entamoeba complex cyst |
0 |
1 |
0 |
3 |
0 |
2 |
2 |
0 |
1 |
0 |
1 |
10 (30.3) |
|
Giardia lamblia cyst |
0 |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 (3.0) |
|
Endolimax nana cyst |
1 |
0 |
0 |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
2 (6.1) |
|
Ascaris lumbricoides ova |
0 |
0 |
0 |
1 |
0 |
1 |
0 |
0 |
1 |
0 |
0 |
3 (9.1) |
|
Trichuris trichiura ova |
0 |
1 |
0 |
2 |
0 |
0 |
0 |
1 |
0 |
0 |
0 |
4 (12.1) |
|
Hymenolepis nana ova |
0 |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 (3.0) |
|
Enterobius vermicularis ova |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
1 |
0 |
0 |
0 |
2 (6.1) |
|
Strongyloides stercoralis rhabditiform larvae |
0 |
1 |
1 |
1 |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
4 (12.1) |
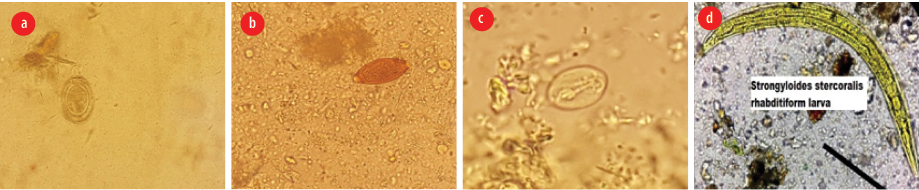
Figure 1: Examples of intestinal parasites detected in the vegetable samples: (a) Hymenolepis nana egg, (b) Trichuris trichiura egg, (c) Giardia lamblia cyst, (d) Strongyloides stercoralis rhabditiform larvae.
Discussion
High incidences of gastrointestinal parasites have been discovered in populations that consume raw fresh vegetables.15 To our knowledge, no studies have been done in the UAE to evaluate the degree of parasitic contamination in fresh vegetable produce with intestinal parasites. Thus, our study is the first in the country to determine the degree of parasitic contamination of some of the frequently eaten raw vegetables from local farms and supermarkets. Protozoan cysts and helminths eggs were detected in 15.1% (33/218) of routinely consumed vegetables. This was similar to reports from Khartoum state, Sudan (13.5%)9, and Riyadh, Saudi Arabia (16.2%).12 Higher parasite contamination rates were reported in Ghana (36%),16 Jose, Nigeria (36%)17, and Khorramabad, Iran (79%).18 Entamoeba complex (E. histolytica/E. dispar/E. moshkovskii) cyst was the most prevalent parasitic stage we detected (30.3%), with spring onions, radish, and broccoli being the most contaminated (22.9%, 29.4%, and 30.0%, respectively) [Table 2]. Likewise, higher rates of E. histolytica/E. dispar were also reported in the Gaza governorate (37.5%),19 and Khartoum state, Sudan (42.9%).9 Lesser rates of E. histolytica infection were reported from Manila, Philippines (0.6%),13 and Eastern Showa, Ethiopia (8.3%).20 It is possible that the Entamoeba complex species detected in those previous studies were the nonpathogenic strains (E. dispar or E. moshkovskii). Both species are morphologically identical to the pathogenic E. histolytica making it paramount that molecular speciation is included in future studies. The wide discrepancy observed in those studies could be attributed to the disparities in the surface shape and texture of the vegetables, the detection method used, different laboratory techniques used, sample size, and the type of the vegetables investigated, geographical location and origin of the vegetables, type of water sprinkled on the vegetables, use of untreated night soil, water quality used for irrigation, modest farming practices, and handling measures after harvesting.9 All these factors play instrumental roles in the epidemiology of parasites transmission.
Reports from other parts of the world revealed lower rates of contamination with T. trichiura eggs - Accra, Ghana (2%),21 Khartoum, Sudan (2.9%),9 Gaza governorate (1.3%)19, and Qazvin province, Iran (0.9%).22 We found a contamination rate of 12.1% with T. trichiura ova with the highest rate in spring onions (50.0%, 2/4). Another soil parasite detected was the A. lumbricoides. Recognized as transmitted in the same mode as T. trichura, fertilized A. lumbricoides eggs were detected in 9.1% of the samples (mainly contaminating leafy vegetables, spring onion, radish, and mint) [Table 2]. This could be attributed to its high level of obduracy and resistance of the parasite eggs.16 Other studies revealed a variation in the rates of A. lumbricoides in vegetables (Ardabil (2%), Qazvin (2.3%), and Tabriz (24%) in Iran, and Southwestern Nigeria (16.7%)).22–25 E. vermicularis ova, however, were identified in 6.1% of our vegetable samples. This result agreed with rates reported in South Western Saudi Arabia (6.3%) and Zahedan, Iran (8.1%).8,26 Much lower percentages of E. vermicularis contamination were reported from Accra, Ghana (2%),21 Benha, Egypt (4.9%),27 Burdur, Turkey (0.9%).28
G. lamblia cysts were detected in 3.0% of the fresh produce with lettuce found to be the only vegetable contaminated with this parasitic protozoan [Table 2]. Other studies reported somewhat similar contamination rates.21,22 Nonetheless, significantly higher rates of G. lamblia cysts were reported from Khartoum, Sudan (22.9%),9 the Gaza governorate, Palestine (28.7%),19 Amman and Baqa’a – two cities in Jordan (23%).29 Similar to both the Egyptian and Jordanian reports, lettuce was the most contaminated vegetable with G. lamblia cysts in our study.27,29 Furthermore, and consistent with a Jordanian study,29 no G. lamblia cysts were seen in cucumber, tomato, and parsley (0.0%). Likewise,
H. nana eggs were detected in 3.0% of the vegetables analyzed with lettuce again being the main type of vegetable contaminated with this helminth. Similar rates and vegetable type (lettuce) harboring H. nana ova were reported from Alexandria, Egypt (2.6%), and Mazandaran province, Iran (2.2%).10,30 In Qazvin, Iran, H. nana eggs were detected in 0.5% only and was the least parasite contaminating the green vegetables.22
S. stercoralis rhabditiform larvae were detected in 12.1% of the examined raw produce with lettuce, radish, spring onions, and watercress being the main vegetables contaminated with the larvae of this facultative intestinal nematode. Incongruent contamination rates with this larvae form have been reported from various regions in vegetables like those in our study (lettuce, spring onion, and cabbage).19,21,22,25 A significant number of larvae and adult nematodes were detected and consequently disregarded since their diagnostic features differ from those infecting humans. Non-virulent and a commensal E. coli cyst was the second-highest identified parasitic contaminant (18.2%). This was comparable to contamination rates noted in the South Western part of Saudi Arabia and Khartoum, Sudan where prevalences of 19.04% and 14.3%, respectively, were reported.9,26 Moreover, Endolimax nana, another commensal, was found in 6.1% of the vegetable produce. It is important to point out that the presence of both these protozoa is an indication of the contamination of vegetables with human waste.
Major limitations of our study included failure to test the water samples used by many supermarkets, vendors and local farms to sprinkle on their produce for the presence of parasites, the small size of the samples investigated, failure to examine the local soil, sewage and irrigation water for the different parasitic stages of human and animal origin, and the inability to assess and distinguish viable helminths eggs from non-viable ones and ascertain their infectiousness.
Although statistical analysis revealed no significant association between the vegetable type and the parasite occurrence (p > 0.050) nor a dependency of the parasite incidence with the vegetable type (p > 0.050), our study highlights the potential of fresh raw produce serving as sources of infection with countless human pathogens. Like other studies, vegetables such as lettuce, spring onions, watercress, and parsley (possessing uneven surfaces facilitating easy parasitic stage attachment) have been shown to have the highest parasite contamination as opposed to smooth-surfaced vegetables.21,27 Another important finding is the need for proper washing and handling of vegetables since a significant number of both vegetables and fruits are imported from developing countries where environmental conditions and hygiene practices are compromised. The uncontrolled use of natural manure containing excrement from both humans and animals, and the use of sewage water for irrigations are some of the practices in use in those countries.5,25,27
Conclusion
Our study highlights the possibility of raw vegetables, especially the leafy ones, serving as an important cause of foodborne disease outbreaks. Our results also stress the urgent need for public education on safe and proper handling of fresh vegetables such as educating the public on how to properly disinfect these vegetables before eating them fresh or slightly cooked.
Disclosure
The authors declared no conflict of interest. No funding was received for this study.
references
- 1. Kniel KE, Lindsay DS, Sumner SS, Hackney CR, Pierson MD, Dubey JP. Examination of attachment and survival of toxoplasma gondii oocysts on raspberries and blueberries. J Parasitol 2002 Aug;88(4):790-793.
- 2. Sunil B, Thomas DR, Latha C, Shameem H. Assessment of parasitic contamination of raw vegetables in Mannuthy, Kerala state, India. Vet World 2014 Apr;7(4):253-256.
- 3. Alade GO, Alade TO, Adewuyi IK. Prevalence of intestinal parasites in vegetables sold in Ilorin, Nigeria. Am-Eurasian J Agric Environ Sci 2013;13(9):1275-1282.
- 4. ul-Haq S, Maqbool A, Khan UJ, Yasmin G, Sultana R. Parasitic contamination of vegetables eaten raw in Lahore. Pakistan. J Zool (Lond) 2014;46(5):1303-1309.
- 5. Pires SM, Vieira AR, Perez E, Lo Fo Wong D, Hald T. Attributing human foodborne illness to food sources and water in Latin America and the Caribbean using data from outbreak investigations. Int J Food Microbiol 2012 Jan;152(3):129-138.
- 6. Olyaei A, Hajivandi L. Parasitological contamination of markets and farms in vegetables consumed in southern Iran. Glob Vet 2013;10(3):327-331.
- 7. Tefera T, Biruksew A, Mekonnen Z, Eshetu T. Parasitic contamination of fruits and vegetables collected from selected local markets of Jimma town, southwest Ethiopia. Int Sch Res Not 2014 Aug;382715.
- 8. Ebrahimzadeh A, Jamshidi A, Saeed Mohammadi S. The parasitic contamination of raw vegetables consumed in Zahedan, Iran. Health Scope 2013 Jan;1(4):205-209.
- 9. Mohamed MA, Siddig EE, Elaagip AH, Edris AM, Nasr AA. Parasitic contamination of fresh vegetables sold at central markets in Khartoum state, Sudan. Ann Clin Microbiol Antimicrob 2016 Mar;15:17.
- 10. Said D. Detection of parasites in commonly consumed raw vegetables. Alex J Med 2102 Dec;48(4):345-352.
- 11. Tomass Z, Kidane D. Parasitological contamination of wastewater irrigated and raw manure fertilized vegetables in Mekelle city and its suburb, Tigray, Ethiopia. CNCS Mekelle Univ 2012;4(1):77-89.
- 12. Al-Megrin WA. Prevalence of intestinal parasites in leafy vegetables in Riyadh, Saudi Arabia. Int J Trop Med 2010;5(2):20-23.
- 13. Sia Su GL, Mariano CM, Matti NS, Ramos GB. Assessing parasitic infestation of vegetables in selected markets in Metro Manila, Philippines. Asian Pac J Trop Dis 2012 Feb;2(1):51-54.
- 14. Idahosa OT. Parasitic contamination of fresh vegetables sold in Jos markets. Glob J Med Res 2011 May;11(1):21-25.
- 15. Srikanth R, Naik D. Health effects of wastewater reuse for agriculture in the suburbs of Asmara city, Eritrea. Int J Occup Environ Health 2004 Jul-Sep;10(3):284-288.
- 16. Amoah P, Drechsel P, Abaidoo RC, Ntow WJ. Pesticide and pathogen contamination of vegetables in Ghana’s urban markets. Arch Environ Contam Toxicol 2006 Jan;50(1):1-6.
- 17. Damen JG, Banwat EB, Egah DZ, Allanana JA. Parasitic contamination of vegetables in Jos, Nigeria. Ann Afr Med 2007 Sep;6(3):115-118.
- 18. Ezatpour B, Chegeni AS, Abdolahpour F, Azami M, Alirezaei M. Prevalence of parasitic contamination of raw vegetables in Khorramabad, Iran. Food Control 2013 Nov;34(1):92-95.
- 19. Al-Shawa RM, Mwafy SN. The enteroparasitic contamination of commercial vegetables in Gaza Governorates. J Infect Dev Ctries 2007;1(1):62-66.
- 20. Benti G, Gemechu F. Parasitic contamination on vegetables irrigated with Awash river in selected farms, eastern Showa, Ethiopia. J Parasitol Vector Biol 2014 Jul;5(7):103-109.
- 21. Duedu KO, Yarnie EA, Tetteh-Quarcoo PB, Attah SK, Donkor ES, Ayeh-Kumi PF. A comparative survey of the prevalence of human parasites found in fresh vegetables sold in supermarkets and open-aired markets in Accra, Ghana. BMC Res Notes 2014 Nov;7:836.
- 22. Shahnazi M, Jafari-Sabet M. Prevalence of parasitic contamination of raw vegetables in villages of Qazvin Province, Iran. Foodborne Pathog Dis 2010 Sep;7(9):1025-1030.
- 23. Daryani A, Ettehad GH, Sharif M, Ghorbani L, Ziaei H. Prevalence of intestinal parasites in vegetables consumed in Ardabil, Iran. Food Control 2008 Aug;19(8):790-794.
- 24. Balarak D, Ebrahimi M, Modrek MJ, Bazrafshan E, Mahvi AH, Mahdavi Y. Investigation of parasitic contaminations of vegetables sold in markets in the city of Tabriz in 2014. Glob J Health Sci 2016 Oct;8(10):54811.
- 25. Ogbolu DO, Alli OA, Ogunleye VF, Olusoga-Ogbolu FF, Olaosun I. The presence of intestinal parasites in selected vegetables from open markets in south western Nigeria. Afr J Med Med Sci 2009 Dec;38(4):319-324.
- 26. Al-Binali AM, Bello CS, El-Shewy K, Abdulla SE. The prevalence of parasites in commonly used leafy vegetables in South Western, Saudi Arabia. Saudi Med J 2006 May;27(5):613-616.
- 27. Eraky MA, Rashed SM, Nasr Mel-S, El-Hamshary AM, Salah El-Ghannam A. Parasitic contamination of commonly consumed fresh leafy vegetables in Benha, Egypt. J Parasitol Res 2014;2014(Jun):613960.
- 28. Adanir R, Tasci F. Prevalence of helminth eggs in raw vegetables consumed in Burdur, Turkey. Food Control 2013 Jun;31(2):482-484.
- 29. Ismail Y. Prevalence of parasitic contamination in salad vegetables collected from supermarkets and street vendors in Amman and Baqa’a – Jordan. Pol J Microbiol 2016;65(2):201-207.
- 30. Rostami A, Ebrahimi M, Mehravar S, Fallah Omrani V, Fallahi S, Behniafar H. Contamination of commonly consumed raw vegetables with soil transmitted helminth eggs in Mazandaran province, northern Iran. Int J Food Microbiol 2016 May;225:54-58.