Cystic echinococcosis (CE) is a zoonotic infection caused by the tapeworm of the genus Echinococcus. Humans act as the intermediate host and infected patients can remain asymptomatic for many years until they present with a slowly growing cystic mass in the liver, lung, or any other organ.1
Echinococcosis is one of the 17 neglected tropical diseases recognized by the World Health Organization (WHO) and a diagnosis of pulmonary CE can be especially challenging as it can present with nonspecific signs and symptoms.1 Surgical resection and medical therapy with albendazole are the currently recommended modalities for treatment.1 However, there is little evidence to support one modality of treatment over the other. Below we present two cases of pulmonary CE caused by E. granulosus that have been treated with surgical and medical therapy with good clinical outcomes.
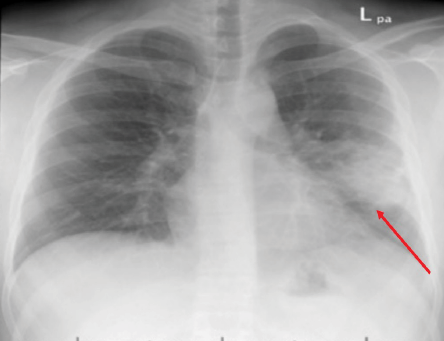
Figure 1: Chest X-ray of case one showing left lung mid-zone opacity with ill-defined margins (red arrow).
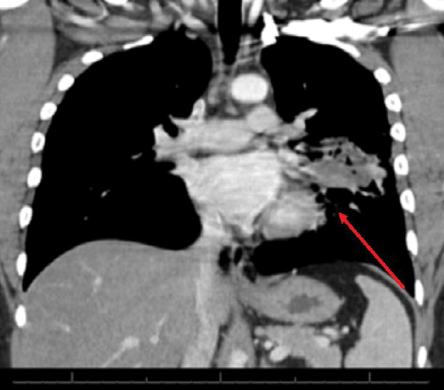
Figure 2: Computed tomography image of the chest showing a left lung mid-zone opacity with ill-defined margins with surrounding lung parenchymal changes (red arrow).
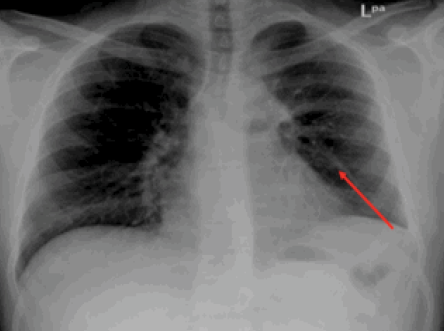
Figure 3: Chest X-ray showing radiological improvement of previously seen left lung mid-zone opacity (red arrow) after 28 days of treatment with albendazole.
Case report
Case one
In November 2011, a 21-year-old male was referred from a rural hospital to Sultan Qaboos University Hospital. He had previously been well until a month ago when he had developed a productive cough with yellow sputum associated with chest pain, dyspnea, intermittent fevers, and 10 kg unintentional weight loss. He denied any hemoptysis or night sweats, history of travel, known tuberculosis contact or a family history of malignancy, or tobacco use. He lived in town but spent many of his weekends on his family’s farm with significant livestock exposure.
Upon initial presentation at the rural hospital, his chest X-ray showed an area of cavitation with an air fluid level. He was started on broad spectrum antibiotics and referred to our institute for further evaluation. Unfortunately, he was lost to follow-up before further work-up could be completed. He then presented again in May 2013 with intermittent hemoptysis and continued weight loss. All laboratory studies were within normal limits. An X-ray [Figure 1] and computed tomography (CT) of his chest [Figure 2] showed a left lung mid-zone opacity with ill-defined margins with surrounding lung parenchymal changes. A bronchoscopy was performed, which showed thick white secretions from the left lingular bronchus. Sputum, blood, bronchoalveolar lavage fluid, and bacterial and mycobacterial cultures were all negative as well as QuantiFERON test.
Due to an unrevealing work-up and malignancy concerns, the patient underwent a left upper lobectomy due to intraoperative findings of left upper lobe involvement. The histopathology report was negative for malignant cells but showed a cavity with a degenerated worm consistent with the diagnosis of pulmonary EC. An ultrasound of the abdomen was performed to search for concomitant liver cysts, which was negative. The patient was started on a 28-day course of adjuvant albendazole 400 mg twice daily to prevent relapse.
The patient followed closely and he had complete resolution of his symptoms and radiological improvement in his chest X-ray [Figure 3].
Case two
A 44-year-old male presented to the pulmonology clinic at Sultan Qaboos University Hospital with a two-month history of productive cough without fever or hemoptysis. He denied weight loss, tuberculosis contact, travel, or family history of malignancy. He was a heavy ex-smoker with no other relevant medical history and was working as a software programmer in the city of Salalah, Oman.
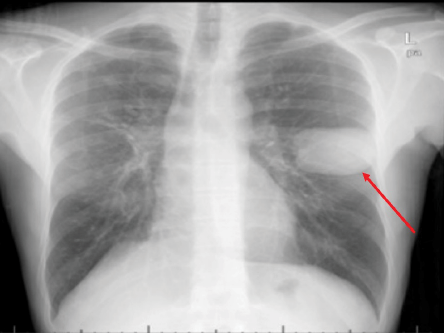
Figure 4: Chest X-ray of a 44-year-old male showing a well-defined homogeneous round opacity of 67 mm × 48 mm in the mid-zone of the left lung (red arrow).
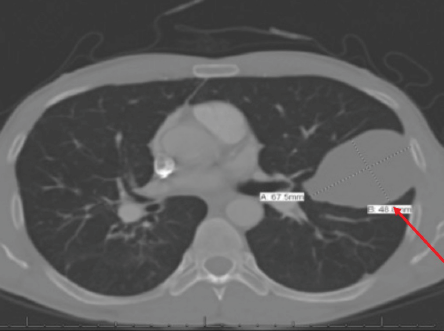
Figure 5: CT scan of the chest of a 44-year-old male showing a unilocular unifocal cyst of 67 mm × 48 mm (red arrow) in the mid-zone of the left lung with fibrotic changes.
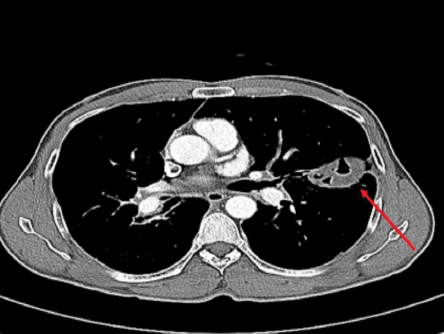
Figure 6: Computed tomography scan of the chest of a 44-year-old male showing improvement in previously seen cyst (red arrow).
A review of his previous imaging (a chest X-ray and CT scan) from two months earlier showed a 46 mm fissure based thin-walled cyst with underlying subsegmental collapse. At that time, he was treated as a case of bacterial lung abscess. However, despite completing a course of antibiotics, he remained symptomatic.
Upon presentation, his physical examination was unremarkable. All laboratory investigations were within normal limits except for mild eosinophilia. A chest X-ray showed a well-defined homogeneous round opacity of 67 mm × 48 mm in the mid-zone of the left lung with some fibrotic changes and CT of his chest and abdomen showed a left pulmonary unilocular unifocal cyst with multiple right hepatic lobe cysts [Figure 4 and 5]. Based on the highly suggestive radiological findings, an anti-Echinococcus antibody was tested and found to be positive with a titer of 1/256. After delving more into the patient’s background, it was found that he had some interaction with livestock, mostly goats.
The patient was advised operative excision of the cyst but refused, so medical management was started with a three-month course of albendazole 400 mg twice daily and close radiological surveillance. His treatment course was largely uneventful, apart from one episode of severe violent coughing and exportation of a large amount of watery whitish material. A chest X-ray and CT scan after the episode showed a markedly reduced size of the left lung cystic lesion, which had become predominantly filled with air.
The patient completed his three-month course of albendazole with radiological improvement [Figure 6] and declining titers of anti-Echinococcus antibody (which had decreased to 32).
Discussion
It was previously thought that E. granulosus was the only causative agent of CE. However, the advancement of phylogenetic systematics has resulted in the recognition of E. equinus, E. ortleppi, and E. canadensis as additional causative pathogens of CE. CE is a cestode infection endemic throughout most of the Middle East and North Africa.1 However, in a serological survey of EC in Oman, its prevalence was considerably lower than the regional average prevalence of EC in human (0.3%) and camel (1.5%) populations. The low prevalence of CE in Oman was attributed to the general lack of domesticated dogs due to cultural and religious beliefs with livestock being traditionally free grazing.2 Canines are the definitive hosts that become infected by ingesting meat contaminated with hydatid cysts. Inside the canines, the cysts mature into adult worms that produce eggs that are expelled in the stool. Intermediate hosts (sheep, cattle, goats, or pigs) and incidental hosts (humans) acquire the infection by ingesting food or water contaminated with eggs excreted from canine feces. Following ingestion of the egg, the larva penetrates the intestinal wall and travels to any of several target organs and develops into a hydatid cyst. The liver is the most commonly affected organ, followed by the lungs (25% of cases). Concomitant hepatopulmonary hydatid disease is reported in 34.8% of cases.1
Diagnosing pulmonary EC can be troublesome in countries where canine exposure is limited as pulmonary EC can present with an array of nonspecific local and systemic manifestations such as cough (53–62%), chest pain (49–91%), dyspnea (10–70%), and hemoptysis (12–21%).3 Less frequent symptoms include malaise, nausea and vomiting, and thoracic deformities. It may also present as a slow-growing cystic mass that can invade adjacent tissues causing mass effect and metastasize to remote organs, and mimicking a malignant neoplasm. Additionally, it is not uncommon for cysts to be diagnosed as incidental radiographic findings, as it may be asymptomatic in children and younger adolescents due to the higher elasticity of lung parenchyma. Complications of pulmonary EC include secondary bacterial infection and cyst rupture into an adjacent bronchus which may manifest by expectoration of sputum consisting of larval tissue and fragments of the laminated membrane as may have occurred in patient number two.3
Radiographic imaging of the chest is the most helpful diagnostic modality. After the discovery of a pulmonary hydatid cyst, imaging of the abdomen should be performed, which can reveal liver involvement in 15% of cases. The finding of concomitant lung and liver cyst can be a clue to the diagnosis of EC.3,4
Immunodiagnostic methods may aid the diagnosis but lack specificity and sensitivity and have significant cross-reactivity with other helminthic infections. The WHO and World Organization for Animal Health recommend an initial screening testing with either enzyme-linked immunosorbent assays, indirect hemagglutination antibody tests, latex agglutination, immunofluorescence antibody tests, and immunoelectrophoresis.5 These initial tests are positive in 65% of pulmonary CE.6 Confirmatory testing after an initial screening test can be performed by immunoblot assays to test for reactivity with E. granulosus antigen subunits.6 If positive this can also be useful for monitoring disease progression. Immunoglobulin E and eosinophil count are nonspecific markers that may become raised after rupture/leakage of the cyst.
Current available treatment modalities for pulmonary CE include watchful waiting, medical therapy, or surgery (often combined with perioperative anti-parasitic therapy). Surgery classically is the gold standard for the management of pulmonary CE.3 The WHO advocates the use of the radiological cyst staging system to assess the need for surgery. The WHO characterizes echinococcosis cysts by the size and radiological appearance.7 On imaging, cysts may appear as unilocular, multilocular with daughter cysts, degenerating (which can be either having floating membranes or solid with daughter cysts), and lastly inactive cyst which often calcified or echogenic. Stage CE1 and CE3, a cyst (which have a single compartment) or cysts < 5 cm in diameter can be treated with medical therapy alone. Larger symptomatic cyst requires surgical removal. For inoperable cysts, the puncture, aspiration, injection, and respiration procedure can also be used.
However, to date, there is no adequate clinical evidence to support the best modality of treatment and recommendations are based on expert recommendations.4
In case one, bronchoscopy revealed active white secretions from the affected lobe; hence, lobectomy was deemed the most relevant choice management with adjuvant chemotherapy, which has been documented to reduce the chances of seeding and recurrence.4 Patient two refused surgical intervention. In cases where surgery cannot be performed (or is unwanted), WHO guidelines recommend several months of albendazole therapy.3,8,9
Conclusion
The above cases highlight the diagnostic difficulty that pulmonary EC can cause, especially when it presents as an isolated lung cyst, which has a wide and variable differential diagnosis. A high index of suspicion is needed for diagnosis, especially in cases of lung abscess not responding to conventional antimicrobial therapy. Both medical and combined medical and surgical therapies have been used in the above-presented cases with good efficacy and a favorable outcome.
Disclosure
The authors declared no conflicts of interest.
references
- 1. Moro P, Schantz PM. Echinococcosis: a review. Int J Infect Dis 2009 Mar;13(2):125-133.
- 2. Idris MA, Ruppel A, Gehrig-Feistel H, Alansari AS, al-Rejaibi AK, Tageldin MH, et al. The seroprevalence of cystic hydatidosis in Oman. Ann Trop Med Parasitol 1999 Apr;93(3):259-263.
- 3. Santivanez S, Garcia HH. Pulmonary cystic echinococcosis. Curr Opin Pulm Med 2010 May;16(3):257-261.
- 4. Brunetti E, Kern P, Vuitton DA; Writing Panel for the WHO-IWGE. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop 2010 Apr;114(1):1-16.
- 5. Agudelo Higuita NI, Brunetti E, McCloskey C. Cystic echinococcosis. J Clin Microbiol 2016 Mar;54(3):518-523.
- 6. Biava MF, Dao A, Fortier B. Laboratory diagnosis of cystic hydatic disease. World J Surg 2001 Jan;25(1):10-14.
- 7. WHO Informal Working Group. International classification of ultrasound images in cystic echinococcosis for application in clinical and field epidemiological settings. Acta Trop 2003 Feb;85(2):253-261.
- 8. Stamatakos M, Sargedi C, Stefanaki Ch, Safioleas C, Matthaiopoulou I, Safioleas M. Anthelminthic treatment: an adjuvant therapeutic strategy against Echinococcus granulosus. Parasitol Int 2009 Jun;58(2):115-120.
- 9. Eckert J, Gemmell MA, Meslin FX, Pawłowski ZS, editors.WHO/OIE manual on echinococcosis in humans and animals: a public health problem of global concern. World Organization for Animal Health (Office International des Epizooties), Paris, France, and World Health Organization, Geneva, Switzerland; 2001.