|
Abstract
Objectives: Heart velocity may be influenced by gagging. The medulla oblongata receives the afferents of gag reflex. Neuronal pools of vomiting, salivation and cardiac parasympathetic fibers are very close in this area. So, their activities may be changed by spillover from each other. Using the heart rate variability (HRV) analysis, the effect of gagging on cardiac sympatovagal balance was studied.
Methods: ECG was recorded from 12 healthy nonsmoker volunteer students for 10 minutes in the sitting position between 10 and 11 AM. Gagging was elicited by tactile stimulation of the posterior pharyngeal wall. At 1 kHz sampling rate, HRV was calculated. The mean of heart rate at low and high frequencies (LF: 0.04-0.15; HF: 0.15-0.4 Hz) were compared before and after the stimulus.
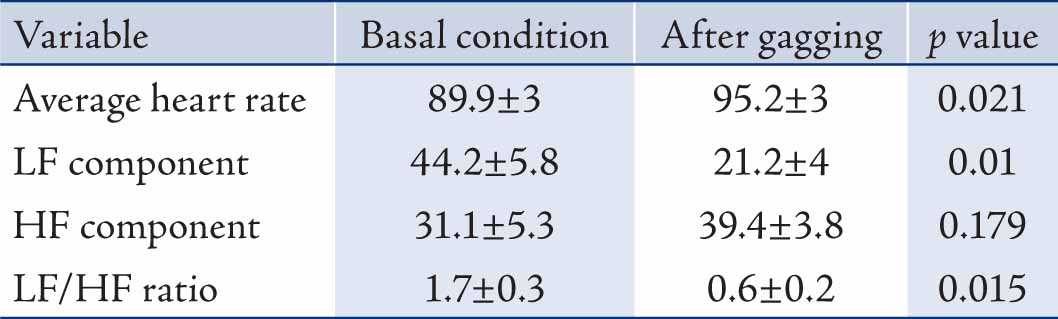
Results: The mean of average heart rate, LF and HF in normalized units (nu) and the ratio of them (LF/HF) before and after the gagging were 89.9 ± 3 and 95.2 ± 3 bpm; 44.2 ± 5.8 and 21.2 ± 4; 31.1 ± 5.3 and 39.4 ± 3.8; and 1.7 ± 0.3 and 0.6 ± 0.2 respectively.
Conclusion: Gagging increased heart velocity and had differential effect on two branches of cardiac autonomic nerves. The paradoxical relation between average heart rate and HRV indexes of sympatovagal tone may be due to unequal rate of change in autonomic fiber activities which is masked by 5 minutes interval averaging.
Keywords: Gag reflex; HRV.
Introduction
The gag reflex protects the pharynx, larynx and trachea against foreign materials. This reflex is usually elicited by tactile stimulation of the posterior pharyngeal wall and some other intraoral structures. In some cases nontactile (visual, auditory, and olfactory) stimuli or some emotions can also trigger this reflex.1
Different visceral afferents can affect the cardiac sympatovagal balance. Nucleus tractus solitarious receives these signals, integrates them and sends efferent commands to the cardiovascular system. The autonomic control of salivation and vomiting are very close to neuronal pools of cardiac autonomic center in the medulla oblongata.2-4 These central commanders can be directed by feedback or feedforward pathways which may cause different crosstalk in the autonomic system like post micturition syncope.5 Gagging may also be accompanied by unpleasant sensation and can lead to fainting, panic attack or nausea.1 There are few reports regarding the cardiac manifestation of gag reflex which potentially may be important in severe gagging, so the effect of gag reflex on heart velocity and sympatovagal tone of sinus node was studied.
Methods
The participants were 12 male healthy nonsmoker volunteer university students' who all signed an informed consent. Persons who had a history of problematic gagging were excluded. All tests were done between 10 and 11 AM and at least 2 hours after meal. The gag reflex was elicited by tactile stimulation of the posterior pharyngeal wall using a cotton cover swab in sitting position. Respiration and ECG were recorded continuously by PowerLab recorder (8/30 ML870 and Dual Bio/Stim ML 408, ADInstrument Ltd. Australia). The sampling rate was 1 kHz. The lead II ECG signals were used for data analysis in two successive 5 minutes before and after the stimulus. HRV was measured in time domain and frequency domain methods. For overcoming the effect of total power inequality on absolute value of LF and HF components, the spectral densities were normalized on the basis of very low frequency component (0-0.04). The normalized value of LF and HF were calculated according the following equation: LF or HF [ms2]/ [total power [ms2]-VLF [ms2] and were used for statistical analysis.6 The mean of heart rate at low and high frequencies (LF: 0.04- 0.15; HF: 0.15-0.4 Hz) were compared before and after the stimulus.
Results
The mean±S.E.M of age, weight and height of subjects were 20±1.2 years, 68±5 kg and 167±6 cm, respectively. Table 1 shows the heart rate, LF and HF in normalized unit (nu) and the LF/HF ratio at rest and following gagging stimulation.
Table 1: The heart rate variability indexes following gagging (mean±S.E.M; n=12).
Discussion
Gagging caused statistically significant increase in average heart rate but illustrated the changing trend of autonomic tone in favor of parasympathetic division over 5 minutes interval. Despite some increase in parasympathetic activity (not significant), there was about 50% reduction in normalized LF and LF/HF ratio. The paradoxical relation between average heart rate and HRV indexes may be due to unequal rate of change in autonomic fiber activities which was masked by 5 minutes averaging following gag stimulation.
The right vagus nerve innervates the sinoatrial node, and parasympathetic hyperstimulation predisposes those affected to bradyarrhythmias; the left vagus nerve when hyperstimulated predisposes the heart to atrioventricular (AV) blocks, but these facts do not affect our results because we recorded average heart rate variability for 5 minutes before and after gagging. It is unclear whether parasympathetic response leads to reflex bradycardia or not.7 There are some reports concerning paradoxical effects of parasympathetic stimulation on heart rate and blood pressure. For example, Gupta et al. reported that laryngoscopy as a trigger of gag reflex can lead to both increase and decrease in heart rate and systolic blood pressure.8 On the other hand, our results showed increase in average heart rate during 5-minute recording, not immediately after gagging.
In our knowledge, it is the first report about the effect of gag reflex on sympatovagal balance of sinoatrial node; however the limitations of this study must be considered before any generalization. Sample size, past gagging experiences and probability of previous conditioning, the effect of age and sex on reflex sensitivity, and/or the possible inequality of motor response of reflex in supine and sitting position may be important. It is reported that the gag reflex response is unrelated to patient age and sex.9 Different neuronal centers in medulla oblongata may be affected by gag reflex afferents or other trigger inputs from higher centers. Although some conditions are more prone to gagging such as uncontrolled diabetes, peptic ulcer disease, and sinusitis, learned and/or conditioned gagging must also be noticed.1 Several approaches are used to assess and to treat disruptive gagging including the Gagging Severity Index and Gagging Problem Assessment, local and general anesthesia and cognitive therapy.1,10,11
Conclusion
Overall, the cardiovascular effects of gagging that may potentially be important in some patients are not well known and further study is recommended.
Acknowledgments
This work was financially supported by a grant from Golestan University of Medical Sciences. Sincere thanks are given to all the volunteers and coworkers. The authors do not report any conflict of interest regarding this work.
|