Papillary cystadenocarcinomas (PCAs) are rare low-grade salivary gland tumors histologically characterized by cystic structures with frequent papillary projections, and lined by cuboidal, columnar, or mucus-secreting cells.1–3 This entity was first included in the World Health Organization (WHO) classification in 1991,4 and further reclassified as cystadenocarcinoma in 2005.5 In the more recent 2017 classification, cystadenocarcinoma and mucinous adenocarcinoma have become diagnoses of exclusion and are lumped under adenocarcinoma, not otherwise specified (NOS).6 Such revisions in classifications and potential confusion are further confounded by historical synonyms, including mucus-producing adenopapillary carcinoma, low-grade papillary adenocarcinoma, and malignant papillary cystadenoma.7,8
While classically regarded as a low-grade malignancy, PCAs with clinically and histologically high-grade features have been reported, reflecting the often-underrecognized morphological diversity of this entity.9–12 An association with lymph node metastases and recurrence may exist.9–14 We present an intermediate-grade PCA case with hopes of increasing recognition of the rare possibility of high-grade features in an often-regarded low-
grade malignancy.
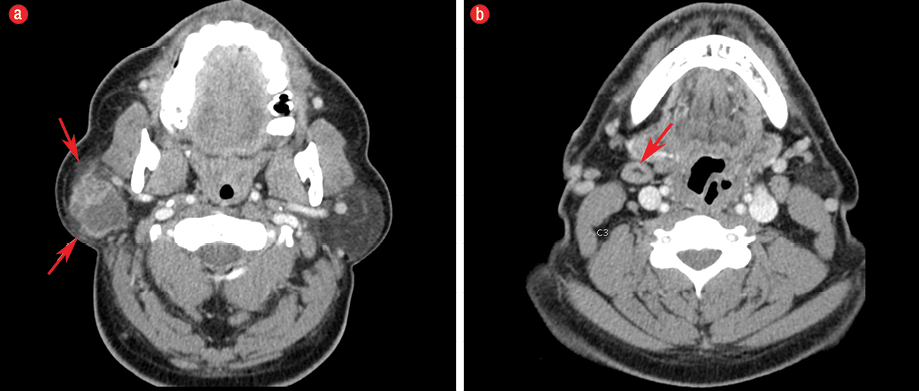
Figure 1: (a) Axial imaging on contrast-enhanced computed tomography at the level of parotid glands demonstrated a 3.3 × 2.8 × 3.2 cm well-defined cystic and solid mass with enhancement of the periphery and internal septa located in the superficial lobe of the right parotid gland. There was mild subcutaneous fat stranding anterior and posterolateral to the parotid mass (red arrows), which likely corresponded to the extraglandular subcutaneous adipose tissue invasion seen on histology. Note the normal appearance of the contralateral fat-predominant parotid gland. (b) Axial imaging slightly inferior at the level of the C3 demonstrates a level IB lymph node measuring approximately larger than 1 cm in the short axis with benign-appearing features including its reniform shape and fatty hilum (red arrow); this was favored to be a reactive lymph node.
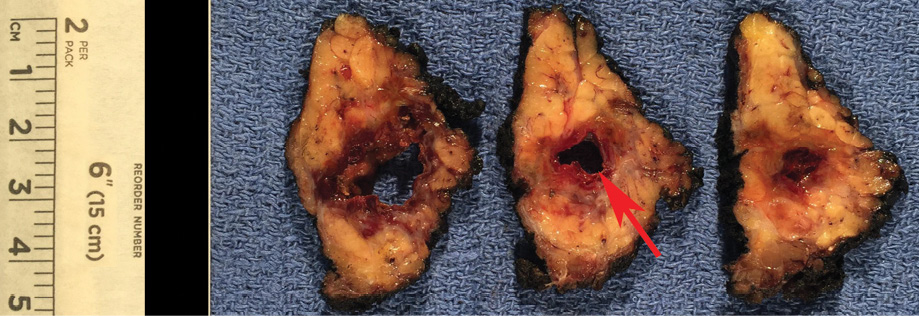
Figure 2: Gross specimen demonstrates a well-marginated, encapsulated mass with a large unilocular cyst (red arrow), and surrounding solid component.
Case report
A 51-year old male presented with progressive right facial fullness that had developed over four years with recent facial pain and serosanguinous drainage. Systems review was negative for fever, fatigue, weight loss, or facial weakness. There was no history of trauma or radiation. Physical examination was notable for a palpable 4 cm right parotid mass. Cranial nerve VII was intact bilaterally and there were no palpable lymph nodes.
Contrast-enhanced computed tomography of the neck demonstrated a 3.3 × 2.8 × 3.2 cm peripherally enhancing cystic and solid mass in the right superficial lobe of the parotid gland [Figure 1]. The patient received a right superficial parotidectomy and selective right neck dissection involving the level II and III lymph nodes.
Gross pathology demonstrated a unilocular cystic mass [Figure 2]. Histopathology demonstrated a large cyst lined by either papillary projections or a single layer of cuboidal cells with mild to moderate atypia and surrounding solid tumor nests with focal cribriform pattern [Figure 3a–c]. No mitosis or necrosis was identified. A focus of lymphovascular invasion [Figure 3d] and multiple foci of stromal invasion [Figure 3b] were noted. Perineural invasion was not identified. There were two foci of invasion into the subcutaneous fibroadipose tissue [Figure 3e]. One of 12 nodes was positive for metastatic carcinoma without extranodal extension [Figure 3f]. We rendered a diagnosis of intermediate-grade PCA in agreement with two additional pathologists.
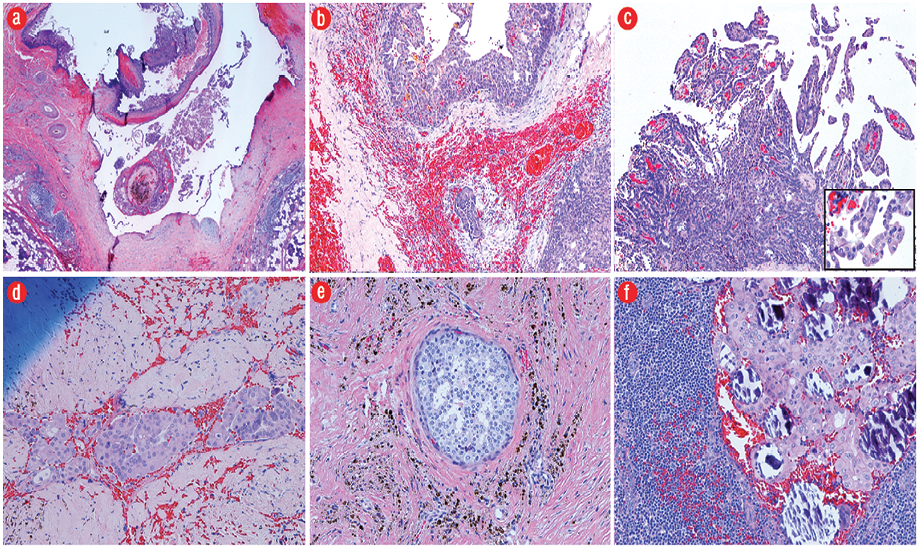
Figure 3: (a) The tumor consisted of predominantly a large cystic space lined by either papillary projections or a single layer of cuboidal cells and surrounding tumor nests (hematoxylin and eosin (H&E) stain, magnification = 2 ×). (b) Infiltrative growth of tumor nests of varying sizes into the fibrotic stroma with hemorrhage (H&E, magnification = 10 ×). (c) Intracystic papillary projections with fibrovascular cores, micropapillary structures without a fibrovascular core, and relatively solid sheets of tumor cells. Neoplastic epithelial cells (insert) demonstrate focal moderate nuclear pleomorphism with prominent nucleoli (H&E, magnification = 20 ×; insert, 40 ×). (d) A focus of tumor with lymphovascular invasion (H&E, magnification = 20 ×). (e) A focus of tumor arranged in cribriform pattern involving the subcutaneous tissue (H&E, magnification = 20 ×). (f) One lymph node positive for metastatic carcinoma with calcification (H&E, magnification = 20 ×).
Table 1: Summary of histopathology cases of intermediate- to high-grade papillary cystadenocarcinoma.
|
Chen et al.*13 |
1990 |
China |
16 P, 1 SM, 5 minor |
37.1† |
17:5
M:F |
“Poorly-differentiated” 12/22 |
High mitotic rate, pleomorphism, disorderly arranged |
8/12 |
3/12 |
N/A |
|
Pollett et al.10 |
1997 |
Canada |
Minor |
80 |
M |
High |
High mitotic rate, pleomorphism, abnormal mitotic figures |
Y |
Y |
Y |
|
Yamada et al.14 |
2007 |
Japan |
SL |
67 |
M |
High |
High mitotic rate, abnormal mitotic figures |
Y |
Y |
N |
|
Koc et al.11 |
2010 |
Turkey |
SM |
74 |
M |
Intermediate |
Pleomorphism, nuclear atypia |
N/A |
N |
N |
Gland: salivary gland involvement; Minor: minor salivary glands; SL: sublingual gland; SM: submandibular gland; P: parotid gland; M: male; F: female; Y: yes; N: no; N/A: not available; LN: lymph node metastases; Ex: extranodal extension.
*Case series of 22 papillary cystadenocarcinomas without individual case-by-case clinical, demographic, and histopathologic findings; †average age of the 22 cases within series.
Table 2: Summary of histological features differentiating high- and low-grade papillary cystadenocarcinomas.
|
Mild pleomorphism |
Marked pleomorphism |
|
Low mitotic rate |
High mitotic rate |
|
Necrosis absent |
Necrosis present |
|
Lymphovascular invasion absent |
Lymphovascular invasion present |
Discussion
PCA was first included in the 1991 WHO classification scheme of salivary gland neoplasms4 and was lumped under the category of adenocarcinoma, NOS in the more recent 2017 WHO classification.6 Papillary was dropped from the name in the 2005 WHO classification, as the intracystic papillary projections were not always a prominent feature.5 The Armed Forces Institute of Pathology originally described PCA as demonstrating prominent cystic features and frequent papillary growth that otherwise lacked features of cystic variants of more common salivary gland malignancies.1 It is often considered the malignant counterpart to the non-Warthin benign cystadenoma, and given the former’s bland nuclear features and lack of atypia, histological differentiation from cystadenoma may often be challenging and largely depends on identifying stromal invasion.15
The rare incidence, terminological revisions, and challenging histological differentiation contribute to the often poorly characterized nature and limited understanding of PCA. The largest study of PCAs was published in a series of 57 cases.2 All tumors demonstrated cystic components and stromal invasion, and approximately 75% of cases demonstrated intraluminal cystic papillary projections. Most cases demonstrated minimal atypia and low mitotic rate (0-1/10 high power field (hpf)), consistent with low-grade features. Of the parotid gland cases, lymph node metastasis was rarely observed and only seen in one case. The tumor in our case demonstrated stromal invasion and predominantly mild with focal moderate nuclear pleomorphism cytologically. The presence of regional lymph node metastasis in our case may have been related to the higher-grade histological features, such as the extra-parenchymal subcutaneous tissue and lymphovascular invasion, which were similarly observed in another case.12
Moreover, while most cases have been considered low-grade, scattered reports of clinically aggressive and histologically high-grade features have been reported [Table 1].9–14 Although there is no universally advocated grading system, high-grade PCAs tend to demonstrate locally aggressive features, cytologic atypia, high mitotic rate, necrosis, and the absence of papillary features [Table 2]. For example, a high-grade PCA demonstrated a mitotic rate of
15–20/10 hpf and nuclear pleomorphism, which were associated with four large metastatic lymph nodes, extranodal extension, and postoperative recurrence (albeit in a setting of not receiving adjuvant radiation therapy).10 Accordingly, another study classified PCAs into well- and poorly-differentiated subtypes, and observed an association between differentiation and nodal metastases and recurrence.13 Lastly, in the previously mentioned case series, those with moderate atypia and higher mitotic rates comprised a larger proportion of those also with lymph node metastases and recurrence.2
While clinical features are neither specific nor reliable in differentiating between high- and low-grade PCAs, certain clinical findings may suggest a malignant entity, such as the presence of a painless mass, lack of tenderness and a degree of fixation to surrounding tissues on palpation, and cranial VII neuropathy.16 The radiological differential diagnosis of a solitary mixed cystic-solid parotid lesion is broad. Considerations include focal presentation of Sjogren syndrome, lymphoepithelial cysts, first branchial cleft cyst, Warthin’s tumor, acinic cell carcinoma, mucoepidermoid carcinoma, salivary duct carcinoma, and metastatic thyroid papillary carcinoma. In most cases, imaging findings of parotid tumors are nonspecific. Low-grade malignant parotid tumors may appear deceivingly nonaggressive and well-defined. Instead, the mainstay of imaging is delineating the extent of the disease preoperatively, which includes evaluating deep or superficial lobe involvement, extraglandular invasion of adjacent structures, perineural invasion, and cervical lymph node metastases. The magnetic resonance imaging findings of an intermediate-grade PCA revealed a well-marginated, heterogeneous mass and with predominantly T1 and T2 prolongation, consistent with mixed cystic-solid components.11 From a histological standpoint, the extent of papillary morphology in PCA is variable. Hence, the pathological differential diagnosis can be broad, and includes salivary duct carcinoma, metastatic thyroid papillary carcinoma, mucoepidermoid carcinoma, papillary cystic variant of acinic cell carcinoma, adenoid cystic carcinoma, intraductal carcinoma, and polymorphous adenocarcinoma.17
The treatment of PCA is complete surgical excision. In general, adequate surgical margins for major salivary gland tumors are recommended and are often > 2 cm when excising high-grade tumors.16 Higher stage and higher-grade parotid tumors, including the presence of an encased functional or impaired facial nerve, are strongly considered for complete parotidectomy. Moreover, neck dissection is recommended for high-grade tumors, those ≥ 4 cm in size, or in cases of clinicoradiological positive nodal disease.16 In many case reports of PCAs, additional postoperative radiation therapy was also considered,14 as in the current case. Considerations for adjuvant radiation therapy are driven by high histological grade, lymphovascular and perineural invasion, nodal metastases, and close surgical margins.16 While PCAs have been reported to foretell a good prognosis,1,2 the intermediate-grade features in the current case, reports of recurrence in prior high-grade PCAs,10,12 and the lack of data available associating clinical outcome with histological features prompted the option of performing adjuvant chemotherapy concurrently with radiation therapy. Ultimately, meaningful conclusions on optimal treatment of specifically PCA are limited by the relative rarity of this entity and few descriptions of this entity in the existing literature.
Conclusion
Although PCA is considered a low-grade carcinoma, both clinically and histologically high-grade features may be seen. No universally advocated grading system exists, but high-grade PCA tends to demonstrate locally aggressive features, cytologic atypia, high mitotic rate, necrosis, and absence of papillary features. An association between histologically high-grade features and lymph node metastases and recurrence may exist, but meaningful conclusions are limited by the low number of studies in the existing literature and the low level of evidence.
Disclosure
The authors declared no conflicts of interest.
references
- Auclair PL, Ellis GL, Gnepp DR, Wenig BM, Janney CG. Salivary gland neoplasms: general considerations. In: Ellis GL, Auclair PL, Gnepp DR, editors. Surgical pathology of the salivary glands. Philadelphia: Saunders; 1991.
- Foss RD, Ellis GL, Auclair PL. Salivary gland cystadenocarcinomas. A clinicopathologic study of 57 cases. Am J Surg Pathol 1996 Dec;20(12):1440-1447.
- Ellis GL. What’s new in the AFIP fascicle on salivary gland tumors: a few highlights from the 4th Series Atlas. Head Neck Pathol 2009 Sep;3(3):225-230.
- Seifert G, Sobin LH. The World Health Organization’s histological classification of salivary gland tumors. A commentary on the second edition. Cancer 1992;70(2):379-385.
- Thompson L. World Health Organization classification of tumours: pathology and genetics of head and neck tumours. Ear Nose Throat J 2006 Feb;85(2):74.
- Seethala RR, Stenman G. Update from the 4th edition of the World Health Organization classification of head and neck tumours: tumors of the salivary gland. Head Neck Pathol 2017;11(1):55-67.
- Blanck C, Eneroth CM, Jakobsson PA. Mucus-producing adenopapillary (non-epidermoid) carcinoma of the parotid gland. Cancer 1971 Sep;28(3):676-685.
- Aloudah NM, Raddaoui E, Aldhahri S, Al-Abbadi MA. Low-grade papillary cystadenocarcinoma of the parotid gland: presentation of a case with cytological, histopathological, and immunohistochemical features and pertinent literature review. Diagn Cytopathol 2009 Feb;37(2):128-131.
- Nakagawa T, Hattori K, Iwata N, Tsujimura T. Papillary cystadenocarcinoma arising from minor salivary glands in the anterior portion of the tongue: a case report. Auris Nasus Larynx 2002 Jan;29(1):87-90.
- Pollett A, Perez-Ordonez B, Jordan RC, Davidson MJ. High-grade papillary cystadenocarcinoma of the tongue. Histopathology 1997 Aug;31(2):185-188.
- Koc M, Yanilmaz M, Yildirim H, Gok U, Cobanoglu B. MRI findings of papillary cystadenocarcinoma of the submandibular gland. Diagn Interv Radiol 2010;16:20-23.
- Khatib Y, Dande M, Patel RD, Kane SV. Cytomorphological findings and histological correlation of papillary cystadenocarcinoma of the parotid: Not always a low-grade tumor. Indian J Pathol Microbiol 2016 Jul-Sep;59(3):368-371.
- Chen XM. [Papillary cystadenocarcinoma of the salivary glands: clinicopathologic analysis of 22 cases]. Zhonghua Kou Qiang Yi Xue Za Zhi 1990;25(2):102-104, 126.
- Yamada S, Matsuo T, Baba N, Rokutanda S, Kawasaki G, Mizuno A, et al. High-grade papillary cystadenocarcinoma of the sublingual gland: a case report. J Oral Maxillofac Surg 2007 Jun;65(6):1223-1227.
- Gallego L, Junquera L, Fresno MF, de Vicente JC. Papillary cystadenoma and cystadenocarcinoma of salivary glands: two unusual entities. Med Oral Patol Oral Cir Bucal 2008 Jul;13(7):E460-E463.
- De Felice F, de Vincentiis M, Valentini V, Musio D, Mezi S, Lo Mele L, et al. Management of salivary gland malignant tumor: the Policlinico Umberto I, “Sapienza” University of Rome Head and Neck Unit clinical recommendations. Crit Rev Oncol Hematol 2017 Dec;120:93-97.
- Telugu RB, Job AJ, Manipadam MT. Papillary cystadenocarcinoma of the parotid gland: a rare case report. J Clin Diagn Res 2016 Jun;10(6):ED01-ED03.