Cardiac surgery is a major surgery associated with significant bleeding. While some authors advocate blood transfusion in severely anemic patients with myocardial injury,1 red blood cell (RBC) transfusion in cardiac surgery was shown to be associated with increased morbidity and mortality.2–4 Myocardial infarction associated with coronary artery bypass graft (CABG) surgeries is a serious complication associated with high
in-hospital stay and long-term mortality.5,6 Increased cardiac troponin I (TI) following cardiac surgery is a highly sensitive and specific indicator of intraoperative ischemic time, and can serve as a marker of an early graft failure following CABG surgery.7,8 High TI levels during the postoperative period can be considered a predictor of unsuccessful short- and long-term outcomes. Moreover, it has been suggested that cardiac TI levels measured 24 hours (TI24) after cardiac surgery is associated with increased postoperative mortality.9 Interestingly, a recent publication showed that RBC transfusion is an independent predictor of TI levels on the first postoperative day following CABG surgery and is associated with a high risk of myocardial infarction.10
Herein, we sought to assess the relationship between blood transfusion and postoperative myocardial infarction in patients who underwent elective cardiac surgery. We also sought to determine the impact of elevated TI24 post-surgery on the development of major postoperative complications and patients’ outcomes.
Methods
We conducted a retrospective review of a prospectively collected data, which included clinical, laboratory, and transfusion details of patients undergoing elective cardiac surgery between 2008 and 2015. A standardized anesthesia protocol was applied on all operated patients. CABG surgery was carried out under cardiopulmonary bypass (CPB), hypothermia at 30–32 ºC, and cardioplegic arrest by antegrade and retrograde multi-dose cold blood cardioplegia to achieve myocardial protection during the aortic cross-clamp. The same heart-lung machine (Stockert Sorin SIII; Sorin Biomedica, Milan, Italy) with a centrifugal pump, a 40 µg arterial filter, and a priming volume of 30 mL/kg body weight of a crystalloid solution (Plasmalyte, Baxter Healthcare) was used for CPB procedures. A membrane oxygenator was used in all cases. All patients were transfused intraoperatively and postoperatively as per the previously described local transfusion and blood management protocols.11,12 Intraoperative allogeneic RBC transfusion was performed aiming for a hematocrit (Hct) level of > 23%. The cell saver (CS) has been in routine use for all patients undergoing cardiac surgery in the hospital since 2010. All CS group patients were transfused with the salvaged RBC in the immediate postoperative period. Allogeneic RBC were transfused perioperatively when the hemoglobin levels fell below 80 g/L or if there were clinical signs of bleeding. Platelets were transfused if signs of bleeding were present with platelet count < 80 × 109/L. Platelets were also given to patients with a history of preoperative aspirin and/or clopidogrel use. Plasma was given if the prothrombin time was more than 1.5 of the upper normal range with prolonged CPB time or clinical signs of bleeding. Local tranexamic acid (TA) was used at the end of the operation for all patients at a dose of 2.5 g of TA in 50 mL of normal saline, injected into the pericardial cavity just before sternal closure. Intravenous TA and other anti-fibrinolytics (including aprotonin) were not used. Postoperative shed mediastinal blood was not processed nor retransfused. In the immediate postoperative period, the patients were transferred to the intensive care unit and extubated once hemodynamic and respiratory stability ensured. Non-invasive ventilation was utilized in the event of respiratory failure post-extubation. A complete blood count was obtained on all patients preoperatively and immediately postoperatively upon arrival to the cardiothoracic intensive care unit. Measurement of TI levels was performed preoperatively, immediately postoperatively, and within the first 24 hours post-surgery. Patients who underwent emergency surgeries and those with preoperative high TI levels of > 6.5 µg/L were excluded.
Patients’ demographics examined included patients’ age, gender, height, weight, and underlying comorbidities. Baseline Hct, platelet count, preoperative TI, and TI24 post-surgery were assessed. Surgical variables assessed included type of surgery and aortic cross-clamp time. Transfusion requirements throughout the intraoperative and the postoperative period (up to 24 hours post-surgery) were assessed. Lastly, rate of in-hospital morbidity including new renal failure, new neurological event, re-exploration surgery, in-hospital length of stay, and in-hospital mortality were reviewed. Variables were presented as means or medians if continuous or proportions if categorical. Levels of TI24 post-surgery was considered elevated when > 6.5 µg/L. Patients were divided into two groups based on their TI24 post-surgery (> 6.5 µg/L vs. ≤ 6.5 µg/L). The baseline characteristics were compared using Student t-test or Mann-Whitney test for continuous variables and chi-squared test for categorical variables. The impact of transfusion on the TI24 post-surgery (> 6.5 µg/L vs. ≤ 6.5 µg/L) was estimated using logistic regression and was adjusted for using the multivariable model that included aortic cross-clamp time and preoperative baseline ejection fraction. Similar analysis was utilized to assess the impact of transfusion on the postoperative myocardial infarction defined by TI levels > 6.5 µg/L and abnormal electrocardiogram changes. In addition, the effect of TI24 post-surgery on the major postoperative complications and outcomes was examined using univariable linear or logistic regression as appropriate in all included patients regardless if receiving blood transfusion. Cross-clamp time was dealt with as a continuous variable. Transfusion was compared as a categorical variable between the high and low troponin groups. Number of RBC units transfused in both groups were also analyzed. Alpha threshold of 0.05 was used for all statistical tests. In the multivariable models, collinearity was assessed using variance inflation factor and none of the variables had value higher than 10. All descriptive and analytical statistics were performed using STATA 13.1 software (StataCorp. 2013. Stata Statistical Software: Release 13. College Station, TX: StataCorp LP).
Results
Total of 542 patients fulfilled our inclusion criteria. Clinical, laboratory, and operative characteristics of studied patients are shown in Table 1. CS was used in 64.0% of patients while 71.1% required RBC transfusion. The same CS was used for all patients in the CS group. Twenty percent of patients had elevated TI24 post-surgery at > 6.5 µg/L (high TI group, Table 1). Using univariable logistic regression analysis, RBC transfusion was found to be associated with high TI24 post-surgery (odds ratio (OR) = 2.33, p = 0.007, 95% confidence interval (CI) = 1.30–4.30). Median RBC transfusion in the low TI24 group was 1 (interquartile range (IQR) 0–2) and in the high TI24 group was 2 (IQR 0–3).
A trend was observed between RBC transfusion and high TI24 post-surgery when aortic cross-clamp time and ejection fraction were adjusted for in the multivariable regression model (OR = 2.06, p = 0.080, 95% CI: 0.90–4.70). Aortic cross-clamp time was associated with high TI24 post-surgery in the multivariable model (OR = 1.01, p = 0.028, 95% CI: 1.00–1.02). Twenty-three percent of patients required platelet transfusion. Platelet transfusion was associated with high TI24 post-surgery (OR = 1.10, p = 0.040, 95% CI: 1.00–1.20). This effect remained significant when aortic cross-clamp time and ejection fraction were adjusted for in the multivariable regression model (OR = 1.13, p = 0.031, 95% CI: 1.01–1.27). Aortic cross-clamp time was also associated with high TI24 post-surgery in this analysis (OR = 1.01, p = 0.023, 95% CI: 1.00–1.02).
Elevated TI24 post-surgery levels were associated with a history of underlying systemic hypertension (OR = 0.59, p = 0.032, 95% CI: 0.36–0.95) and aortic cross-clamp time (OR = 1.01, p = 0.028, 95% CI: 1.00–1.02) in the univariable regression. Age, gender, history of pulmonary hypertension, hypercholesterolemia, angina, myocardial infarction, congestive heart failure, and preoperative Hct level were not found to have any statistically significant association with elevated TI24 post-surgery. Ten percent of the studied cohort developed postoperative myocardial infarction as defined above, while 14 patients died. Postoperative neurological events were encountered in 39 patients, and 33 had new onset renal failure. Re-exploration surgery was performed in 41 patients. The patients had a median in-hospital stay length of eight days (IQR 7–11). Elevated TI24 post-surgery was significantly associated with new renal failure (OR = 2.99, p = 0.004, 95% CI: 1.40–6.32) and increased length of in-hospital stay (OR = 4.50, p = 0.020, 95% CI: 0.69–8.30). No association was found between elevated TI24 post-surgery and the occurrence of a new neurological event (p = 0.074) or increased risk of re-exploration surgery (p = 0.335). Elevated TI24 post-surgery was also found to be significantly associated with higher mortality (OR = 4.15, p = 0.017, 95% CI: 1.29–13.08). When causes of mortality were explored, 50% of patients died postoperatively due to cardiac causes. There was no association between RBC transfusion and postoperative myocardial infarction (p = 0.980).
Table 1: Clinical, laboratory, and operative characteristics of total studied patients (n = 542). Comparison between low TI and high TI groups was made.
|
Age* |
60 (52–66) |
60 (53–67) |
63 (52–66) |
0.614 |
|
Gender |
|
|
|
|
|
Females |
31.0 |
29.0 |
38.0 |
0.105 |
|
Males |
69.0 |
71.0 |
62.0 |
|
Comorbidities |
|
|
|
|
|
Baseline ejection fraction* |
53 (42–61) |
53 (43–61) |
53 (36–60) |
0.209 |
|
Systemic hypertension |
68.0 |
71.0 |
60.0 |
0.092 |
|
Hypercholesterolemia |
66.0 |
68.0 |
62.0 |
0.334 |
|
Angina |
74.0 |
76.0 |
74.0 |
0.855 |
|
Myocardial infarction |
46.0 |
74.0 |
51.0 |
0.240 |
|
Diabetes mellitus |
46.0 |
50.0 |
39.0 |
0.419 |
|
Congestive heart failure |
35.0 |
35.0 |
35.0 |
0.881 |
|
Cerebrovascular disease |
6.6 |
8.0 |
3.0 |
0.204 |
|
Peripheral vascular disease |
3.5 |
4.0 |
1.0 |
0.284 |
|
Surgeries |
|
|
|
|
|
CABG |
72.0 |
77.0 |
59.0 |
< 0.001 |
|
Valve surgery |
14.3 |
12.0 |
15.0 |
|
Combined CABG and valve surgery |
11.0 |
8.0 |
24.0 |
|
Other surgeries |
2.7 |
3.0 |
3.0 |
|
Operative characteristics |
|
|
|
|
|
Cross-clamp time* |
58 (42–85) |
55 (38–74) |
73 (57–101) |
< 0.001 |
|
Investigations |
|
|
|
|
|
Baseline hematocrit†(0.35–0.45) |
39.0 ± 5.0 |
39.0 ± 5.0 |
39.0 ± 5.0 |
0.365 |
|
TI preoperative, µg/L* |
0.03 (0.01–0.08) |
0.02 (0.01–0.07) |
0.03 (0.01–0.09) |
0.149 |
|
TI24 hours postoperative, µg/L* |
2.00 (0.92–5.31) |
1.54 (0.76–2.71) |
11.28 (8.05–20.92) |
< 0.001 |
|
Transfusion requirements |
|
|
|
|
|
RBC transfusion |
71.1 |
69.0 |
84.0 |
0.006 |
CABG: coronary artery bypass graft; RBC: red blood cell; TI: troponin I; TI24: TI levels measured 24 hours.
*Data given as median and interquartile ranges.
†Data given as mean±standard deviation.
Discussion
The decision of blood transfusion in cardiac surgery is affected by many factors and is commonly based on severity of perioperative anemia.13 Different models were developed to predict the need for allogeneic RBC transfusion.11,13–15 However, allogeneic RBC transfusion is known to have important morbidity including the risks of hemolytic transfusion reactions and transfusion associated lung injury. RBC transfusion was shown to be an independent predictor of TI levels on the first postoperative day and was associated with high-risk type V myocardial infarction regardless of the patients’ hemoglobin and Hct levels.10 However, the study included patients who underwent isolated elective off-pump CABG surgery only, and further studies were recommended to verify its findings.
Our results show an association between RBC transfusion and high TI24 post-surgery in univariable logistic regression analysis. However, after adjusting for the aortic cross-clamp time and ejection fraction, only a trend was found. Aortic cross-clamp time was associated with high TI24 post-surgery in the multivariable analyses of RBC and platelet transfusion, suggesting that high TI24 post-surgery likely reflects prolonged surgeries, and other potential intraoperative confounding factors, rather than the transfusion itself [Figure 1]. The lack of association between elevated TI24 post-surgery and the underlying risk factors for ischemic myocardial injuries including age, history of angina, myocardial infarction, and congestive heart failure supports in part this hypothesis. Moreover, high TI24 post-surgery was not found to be associated with preoperative Hct level, while preoperative anemia is proven to be one of the main predictors for RBC transfusion intra- and postoperatively.11,13,15 These findings support that the effect of RBC transfusion in raising the TI24 levels are rather indirect influenced by other factors such as prolonged surgeries, which necessitates longer aortic cross-clamp time and more RBC and platelet transfusion. Both result potentially into myocardial injury, which is represented in high TI24 levels.
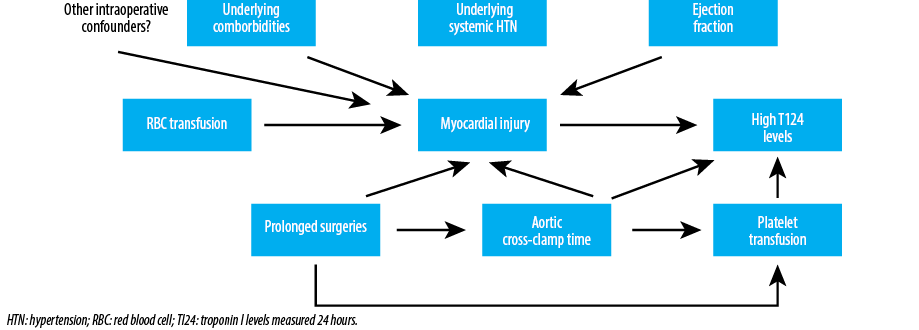
Figure 1: Potential mechanisms to explain the association between RBC transfusion and high postoperative TI24 levels following cardiac surgery.
Studies suggest that cardiac TI is the most accurate biomarker of myocardial injury after cardiac surgery.16 That said, cardiac specific biomarkers, such as TI and CK-MB, are known to be increased following CABG surgeries due to non-ischemic injury to the myocardium secondary to cardiac manipulation or placement on the CPB.17 Attempts were made to define levels of biomarkers that have high sensitivity and specificity of ischemic myocardial injury. One study demonstrated that cut-off values > 6.6 µg/L of TI24 post-surgery had optimal diagnostic performance for the presence of ischemic injury.18 The same cut-off was used in another study to assess the association between RBC transfusion and high TI24 levels post-surgery in CPB.10 In our study, we used the 6.5 µg/L cut-off in combination with the presence of electrocardiogram changes to define ischemic myocardial infarction. It has been shown previously that measurement of preoperative TI levels in patients undergoing CABG surgeries can serve as a marker of increased in-hospital mortality and perioperative myocardial dysfunction.19 To avoid such an effect on the patients’ outcome in our cohort, patients with preoperative elevated TI were excluded from our study.
Our data showed that elevated TI24 post-surgery is associated with an overall poor outcome with higher mortality and morbidity rates compared to patients with non-elevated TI24 post-surgery. This is in line with the results obtained from the large prospective studies that have correlated the postoperative release of cardiac troponin with in-hospital outcomes.20,21 Patients were also found to have increased length of stay in-hospital and higher mortality. The majority of patients died postoperatively due to cardiac causes, and there was no relationship found between RBC transfusion and postoperative myocardial infarction. These results suggest that high TI24 post-surgery likely reflect the complexity of the surgery they underwent and underlying medical condition, which has eventually resulted in higher morbidity and mortality. The effect of other confounding factors cannot be excluded.
Our study has several strengths. To the best of our knowledge, this is the second study that aimed to address the association between transfusion and high TI24 post-surgery, but the first to assess it on a wide range cardiac surgery types. This study utilized a larger cohort size compared to what has been reported previously. That said, the study is limited by its use of retrospective cohort-based methodology, which does not eliminate the effect of other unmeasured confounding factors including the inclusion of severely ill patients with high demand for blood transfusion.
Conclusion
RBC transfusion is associated with increased levels of postoperative TI24 post-surgery in elective cardiac surgeries, albeit a confounding effect cannot be excluded. This effect is not associated with development of myocardial infarction. Larger prospective studies in cardiac and other types of surgeries are needed to confirm our findings.
Disclosure
The authors declared no conflict of interest. No funding was received for this study.
references
- 1. Barbarova I, Klempfner R, Rapoport A, Wasserstrum Y, Goren I, Kats A, et al. Avoidance of blood transfusion to patients suffering from myocardial injury and severe anemia is associated with increased long-term mortality: a retrospective cohort analysis. Medicine (Baltimore) 2015 Sep;94(38):e1635.
- 2. Shaw RE, Johnson CK, Ferrari G, Brizzio ME, Sayles K, Rioux N, et al. Blood transfusion in cardiac surgery does increase the risk of 5-year mortality: results from a contemporary series of 1714 propensity-matched patients. Transfusion 2014 Apr;54(4):1106-1113.
- 3. Reeves BC, Murphy GJ. Increased mortality, morbidity, and cost associated with red blood cell transfusion after cardiac surgery. Curr Opin Anaesthesiol 2008 Oct;21(5):669-673.
- 4. Surgenor SD, Kramer RS, Olmstead EM, Ross CS, Sellke FW, Likosky DS, et al; Northern New England Cardiovascular Disease Study Group. The association of perioperative red blood cell transfusions and decreased long-term survival after cardiac surgery. Anesth Analg 2009 Jun;108(6):1741-1746.
- 5. Schaff HV, Gersh BJ, Fisher LD, Frye RL, Mock MB, Ryan TJ, et al. Detrimental effect of perioperative myocardial infarction on late survival after coronary artery bypass. Report from the Coronary Artery Surgery Study–CASS. J Thorac Cardiovasc Surg 1984 Dec;88(6):972-981.
- 6. McGregor CG, Muir AL, Smith AF, Miller HC, Hannan WJ, Cameron EW, et al. Myocardial infarction related to coronary artery bypass graft surgery. Br Heart J 1984 Apr;51(4):399-406.
- 7. Adams JE III, Bodor GS, Dávila-Román VG, Delmez JA, Apple FS, Ladenson JH, et al. Cardiac troponin I. A marker with high specificity for cardiac injury. Circulation 1993 Jul;88(1):101-106.
- 8. Thielmann M, Massoudy P, Marggraf G, Knipp S, Schmermund A, Piotrowski J, et al. Role of troponin I, myoglobin, and creatine kinase for the detection of early graft failure following coronary artery bypass grafting. Eur J Cardiothorac Surg 2004;26(1):102-109.
- 9. Croal BL, Hillis GS, Gibson PH, Fazal MT, El-Shafei H, Gibson G, et al. Relationship between postoperative cardiac troponin I levels and outcome of cardiac surgery. Circulation 2006 Oct;114(14):1468-1475.
- 10. Biancari F, Kinnunen EM. Red blood cell transfusion is associated with troponin release after elective off-pump coronary artery bypass surgery. Ann Thorac Surg 2012 Dec;94(6):1901-1907.
- 11. Al-Khabori M, Al-Riyami AZ, Mukaddirov M, Al-Sabti H. Transfusion indication predictive score: a proposed risk stratification score for perioperative red blood cell transfusion in cardiac surgery. Vox Sang 2014 Oct;107(3):269-275.
- 12. Al-Riyami AZ, Al-Khabori M, Baskaran B, Siddiqi M, Al-Sabti H. Intra-operative cell salvage in cardiac surgery may increase platelet transfusion requirements: a cohort study. Vox Sang 2015 Oct;109(3):280-286.
- 13. Karkouti K, Wijeysundera DN, Beattie WS; Reducing Bleeding in Cardiac Surgery (RBC) Investigators. Risk associated with preoperative anemia in cardiac surgery: a multicenter cohort study. Circulation 2008 Jan;117(4):478-484.
- 14. Alghamdi AA, Davis A, Brister S, Corey P, Logan A. Development and validation of Transfusion Risk Understanding Scoring Tool (TRUST) to stratify cardiac surgery patients according to their blood transfusion needs. Transfusion 2006 Jul;46(7):1120-1129.
- 15. Ranucci M, Castelvecchio S, Frigiola A, Scolletta S, Giomarelli P, Biagioli B. Predicting transfusions in cardiac surgery: the easier, the better: the Transfusion Risk and Clinical Knowledge score. Vox Sang 2009 May;96(4):324-332.
- 16. Lim CC, Cuculi F, van Gaal WJ, Testa L, Arnold JR, Karamitsos T, et al. Early diagnosis of perioperative myocardial infarction after coronary bypass grafting: a study using biomarkers and cardiac magnetic resonance imaging. Ann Thorac Surg 2011 Dec;92(6):2046-2053.
- 17. Klatte K, Chaitman BR, Theroux P, Gavard JA, Stocke K, Boyce S, et al; GUARDIAN Investigators (The GUARD during Ischemia Against Necrosis). Increased mortality after coronary artery bypass graft surgery is associated with increased levels of postoperative creatine kinase-myocardial band isoenzyme release: results from the GUARDIAN trial. J Am Coll Cardiol 2001 Oct;38(4):1070-1077.
- 18. Pegg TJ, Maunsell Z, Karamitsos TD, Taylor RP, James T, Francis JM, et al. Utility of cardiac biomarkers for the diagnosis of type V myocardial infarction after coronary artery bypass grafting: insights from serial cardiac MRI. Heart 2011 May;97(10):810-816.
- 19. Thielmann M, Massoudy P, Neuhauser M, Knipp S, Kamler M, Marggraf G, et al. Risk stratification with cardiac troponin I in patients undergoing elective coronary artery bypass surgery. European journal of cardio-thoracic surgery 2005;27(5):861-869.
- 20. Carrier M, Pellerin M, Perrault LP, Solymoss BC, Pelletier LC. Troponin levels in patients with myocardial infarction after coronary artery bypass grafting. Ann Thorac Surg 2000 Feb;69(2):435-440.
- 21. Eigel P, van Ingen G, Wagenpfeil S. Predictive value of perioperative cardiac troponin I for adverse outcome in coronary artery bypass surgery. European journal of cardio-thoracic surgery 2001;20(3):544-549.