Polycystic ovary syndrome (PCOS) is a reproductive and metabolic disorder correlated with several risk factors for atherosclerosis.1 Previous studies have shown that the prevalence of cardiovascular disease (CVD) in subjects with PCOS is high compared with the normal population.2,3 Evaluation of preclinical vascular disease using noninvasive tests in patients with PCOS with greater carotid intima-media thickness (CIMT) has demonstrated that there is an increased tendency to atherosclerosis in these patients compared with healthy controls.4,5 Also, some studies have indicated that increased CIMT as a noninvasive marker is a powerful predictor of coronary events and is associated with traditional cardiovascular risk factors, including age, obesity, and metabolic profiles especially increased inflammatory markers.6,7
Antiatherogenic effects such as improving endothelial function, inhibiting platelet aggregation, and reducing levels of triglycerides of omega-3 were previously reported.8 A cross-sectional study observed that higher consumption of omega-3 was associated with a lower prevalence odds of carotid plaques and a lesser thickness of segment-specific CIMT.9 However, no significant effect on CIMT was seen following supplementation with 1 g of omega-3 over 4.9 years in people with CVD and/or CVD risk factors and dysglycemia.10 Several large observational and arterial imaging studies have demonstrated that daily consumption of 100 IU of vitamin E for at least two years decreased atherosclerosis progression and CVD event rates.11,12 However, in another study, vitamin E supplementation (400 IU) in healthy men and women at low risk for CVD did not reduce the progression of CIMT over a three-year period.13
The effects of omega-3 and vitamin E co-supplementation on metabolic profiles have been investigated previously.14,15 Omega-3 and vitamin E co-supplementation seems to work better than single supplementation. The effects of omega-3 and/or vitamin E supplements on human atherosclerosis progression were evaluated in a few small studies in subjects without PCOS, which were inconclusive. The current investigation was, therefore, done to assess the impacts of omega-3 and vitamin E co-supplementation on CIMT and inflammatory factors in patients with PCOS.
Methods
This randomized, double-blind, placebo-controlled clinical trial, registered on the Iranian website for registration of clinical trials (http://www.irct.ir: IRCT201511015623N57) was conducted among 60 subjects with PCOS diagnosed according to the Rotterdam criteria,16 aged 18–40 years old who were referred to the Naghavi Clinic in Kashan, Iran, between June 2016 and October 2016. We excluded pregnant women and those with endocrine diseases and no hormonal treatments in the six months before the study. This trial was approved by the ethics committee of Kashan University of Medical Sciences (KAUMS), and informed consent was taken from all subjects. Subjects were randomly divided into two groups (n = 30 each group) to receive either 1000 mg omega-3 from flaxseed oil containing 400 mg α-linolenic acid plus 400 IU vitamin E supplements or a placebo for 12 weeks. The supplement and placebo (paraffin) were provided by Barij Essence Pharmaceutical Company (Kashan, Iran).
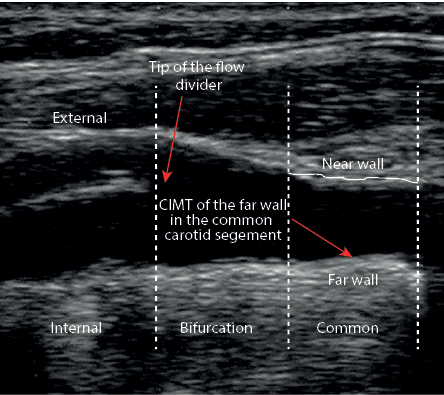
Figure 1: Placement of the ultrasonographic calipers to measure carotid intima-media thickness (CIMT).
To evaluate compliance, we counted the remaining supplements. To increase compliance, all women received short messages every day reminding them to take the capsules.
We considered CIMT as primary outcome measurement and inflammatory parameters as secondary outcomes measurements.
CIMT measurements (maximum and mean of left and right CIMT) were done in patients at the 2 cm distance of the common carotid bifurcation [Figure 1], by the same sonographer, at baseline and after the 12-week intervention using a Doppler ultrasonography device (Samsung Medison V20, Korea) with linear multi-frequencies of 7.5- to 10-MHz probe. Reproducibility information was obtained from the duplicate ultrasound examinations at baseline and the end of the trial. The mean±standard deviation (SD) difference in common CIMT between the two baseline measurements (screening visit and randomization visit) was 0.0004±0.058 mm. The mean absolute mean difference was 0.037±0.042 mm. The intra- and interobserver coefficient variances (CVs) for the repeated measurements of mean CIMT were 6.5% and 9.6%, respectively. Furthermore, the intra- and interobserver CVs for the repeated measurements of maximum CIMT were 7.0% and 10.5%, respectively. All CIMT (mean and maximum thickness) measurements were evaluated blindly by a single experienced ultrasonographer.
Fasting blood samples (10 mm) were collected at baseline and after the 12-week treatment. Serum high-sensitivity C-reactive protein (hs-CRP) values were quantified by an ELISA kit (LDN, Nordhorn, Germany) with inter- and intra-assay CVs of lower than 7.0%. Plasma nitric oxide (NO) values were evaluated using the Griess method17 with inter- and intra-assay CVs < 5.0%.
To establish normal data distribution, we used the Kolmogorov-Smirnov test. To establish differences in anthropometric measures between the two groups, we applied the independent samples t-test. The intention-to-treat (ITT) analysis of the primary study end-point was applied to all randomly allocated subjects. To determine the effects of omega-3 and vitamin E co-supplements on CIMT and inflammatory markers, we used one-way repeated measures ANOVA. To evaluate for several confounders, we adjusted all analyses for baseline values, age, and baseline body mass index (BMI) to avoid potential bias using ANCOVA. A p-value < 0.050 was considered statistically significant. All data entry and statistical analyses were conducted using the SPSS Statistics (SPSS Inc. Released 2009. PASW Statistics for Windows, Version 18.0. Chicago: SPSS Inc.).
We used a randomized clinical trial sample size calculation formula where type one (α) and type two (β) errors were 0.05, and 0.20 (power = 80%), respectively. According to a previous trial,18 we used 0.18 mm as SD and 0.15 mm as the change in mean (effect size) of CIMT as a main variable. Based on the sample size calculation formula, we needed 25 subjects in each group. After considering five dropouts in each group, the final sample size was 30 subjects in each group.
Results
Three patients in the omega-3 and vitamin E co-supplements group and three in the placebo group withdrew from the study due to personal reasons and, therefore, did not complete the trial [Figure 2]. However, all 60 subjects were included in the final analysis using the ITT principle.
There were no significant differences between the two groups in mean age, height, weight, and BMI at baseline, and changes in weight and BMI at the end of the study (data not shown).
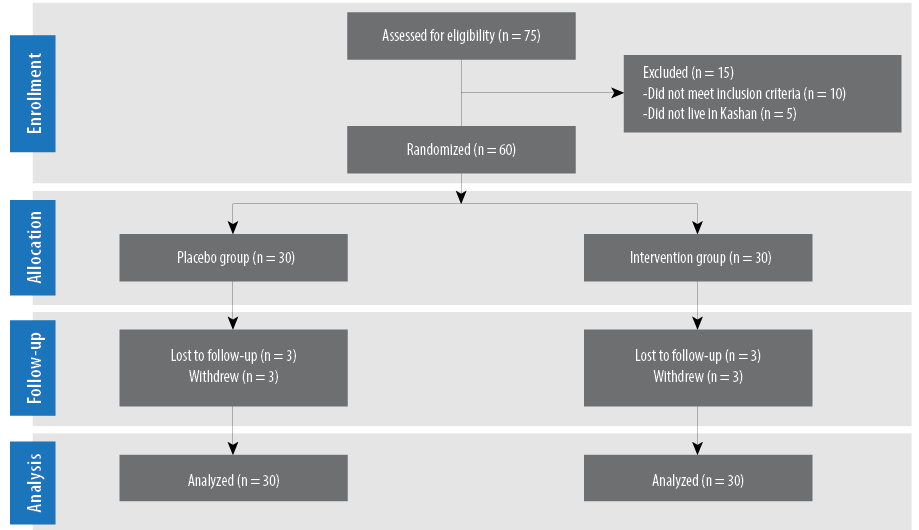
Figure 2: Patient flow diagram.
Omega-3 and vitamin E co-supplementation led to significant decreases in maximum levels of left CIMT (-0.006±0.006 vs. +0.002±0.007 mm, p < 0.001), mean left CIMT levels (-0.005±0.006 vs. +0.002±0.010 mm, p = 0.010), maximum levels of right CIMT (-0.006±0.010 vs. +0.006±0.010 mm, p = 0.010), and mean right CIMT levels (-0.005±0.005 vs. +0.001±0.010 mm, p = 0.020) after the 12-week intervention compared with placebo [Table 1]. Change in hs-CRP (-390.6±942.9 vs. +237.0±754.3 ng/mL, p = 0.006) was significantly different between the intervention and placebo group. We did not observe any significant effect on NO values between the two groups at the end of the study.
There was a significant difference in baseline levels of NO (p = 0.080) between the two groups. When we adjusted the analysis for baseline values of biochemical parameters, age and baseline BMI, mean right CIMT (p = 0.070) became non-significant, while other findings did not alter [Table 2].
Table 1: Carotid intima-media thickness (CIMT) and inflammatory markers at baseline and 12 weeks after the intervention in patients with polycystic ovary syndrome.
|
Mean left CIMT, mm |
0.47 ± 0.04 |
0.47 ± 0.04 |
0.002 ± 0.010 |
0.400 |
0.46 ± 0.02 |
0.45 ± 0.03 |
-0.005 ± 0.006 |
< 0.001 |
0.010 |
|
Maximum left CIMT, mm |
0.56 ± 0.05 |
0.57 ± 0.05 |
0.002 ± 0.007 |
0.050 |
0.55 ± 0.03 |
0.55 ± 0.03 |
-0.006 ± 0.006 |
< 0.001 |
< 0.001 |
|
Mean right CIMT, mm |
0.46 ± 0.03 |
0.46 ± 0.03 |
0.001 ± 0.010 |
0.600 |
0.48 ± 0.05 |
0.47 ± 0.05 |
-0.005 ± 0.005 |
< 0.001 |
0.020 |
|
Maximum right CIMT, mm |
0.56 ± 0.04 |
0.56 ± 0.03 |
0.006 ± 0.010 |
0.040 |
0.57 ± 0.05 |
0.57 ± 0.05 |
-0.006 ± 0.010 |
0.004 |
0.010 |
|
hs-CRP, ng/mL |
2646.7 ± 1492.3 |
2883.7 ± 1488.9 |
237.0 ± 754.3 |
0.090 |
2877.9 ± 2095.5 |
2487.3 ± 1673.1 |
-390.6 ± 942.9 |
0.030 |
0.006 |
Data presented as mean ± standard deviation.
1p-values represent paired-samples t-test.
2p-values represent the time × group interaction (computed by analysis of the one-way repeated measures ANOVA).
hs-CRP: high-sensitivity C-reactive protein; NO: nitric oxide.
Table 2: Adjusted changes in carotid intima-media thickness (CIMT) and inflammatory markers in patients with polycystic ovary syndrome.
|
Mean left CIMT, mm |
0.002 ± 0.002 |
-0.005 ± 0.002 |
0.020 |
|
Maximum left CIMT, mm |
0.003 ± 0.001 |
-0.006 ± 0.001 |
< 0.001 |
|
Mean right CIMT, mm |
0.001 ± 0.002 |
-0.005 ± 0.002 |
0.070 |
|
Maximum right CIMT, mm |
0.005 ± 0.003 |
-0.005 ± 0.003 |
0.007 |
|
hs-CRP, ng/mL |
206.6 ± 140.1 |
-360.2 ± 140.1 |
0.006 |
Data presented as means ± standard deviation. Values are adjusted for baseline values, age, and body mass index at baseline.
1Obtained using ANCOVA.
hs-CRP: high-sensitivity C-reactive protein; NO: nitric oxide.
Discussion
To the best of our knowledge, this study is the first of its kind. We evaluated the beneficial effects of omega-3 and vitamin E co-supplementation on CIMT and inflammatory parameters among patients with PCOS. We found that co-supplementation with omega-3 and vitamin E for 12 weeks in patients with PCOS had beneficial effects on CIMT and hs-CRP levels, but did not affect NO values.
Omega-3 and vitamin E co-supplementation resulted in a significant reduction in maximum and mean levels of left and right CIMT in patients with PCOS compared with placebo. Another study observed an inverse relationship between fetal growth and arterial wall thickness in childhood, which can be prevented by dietary omega-3 supplementation in the first five years of life.19 In addition, supplementation with 3 g/day omega-3 for six months in patients undergoing hemodialysis decreased CIMT.18 The favorable effectiveness of omega-3 on CIMT progression in people with combined hyperlipoproteinemia was also reported.20 Co-supplementation with vitamins E (136 IU/day) and C (250 mg/day) in hypercholesterolemic men for six years reduced common carotid artery (CCA)-IMT.21 Our findings are inconsistent with the results of previous studies examining the effects on carotid and coronary atherosclerosis,22–24 as well as a recent meta-analysis of the effects of omega-3 fatty acids on clinical outcomes.25 In addition, vitamin E supplementation (1200 IU/day) for two years in patients with coronary artery disease reduced parameters of inflammation and oxidative stress, but had no significant effect on CIMT.26 It must be kept in mind that beneficial effects of omega-3 fatty acids or vitamin E on CIMT in most previous studies were observed after six months, while in our study, the beneficial effects of omega-3 and vitamin E co-supplementation on CIMT were seen after three months. This may due to co-supplementation working better than single supplementation. However, data on CVD events in women with PCOS are limited, but a recent meta-analysis demonstrated that women with PCOS had twice the relative risk of CVD or stroke than control patients.27 In a meta-analysis study, CIMT artery in women with PCOS was significantly higher than healthy women. CIMT has been widely used as a surrogate index of atherosclerosis and CVD events.28,29 Omega-3 may mitigate the adverse effects of reduced Δ-5 desaturase activity,19 which in turn may improve CIMT. Moreover, omega-3 supplementation appears to have a cardioprotective effect in adults, potentially through effects on lipid fractions, endothelial function, and/or antiarrhythmic and anti-inflammatory actions.30 Vitamin E intake due to its antioxidant and anti-inflammatory effects may improve CIMT. 21
Omega-3 and vitamin E co-supplementation in patients with PCOS for 12 weeks was associated with a significant reduction in hs-CRP levels, but unchanged NO values. Supporting our results, Rizza et al,31 observed that supplementation with omega-3 (2 g/day) significantly improved endothelial function and decreased pro-inflammatory markers in offspring of patients with type 2 diabetes mellitus for 12 weeks. In addition, another meta-analysis study observed that marine-derived omega-3 supplementation had a significant CRP lowering effect.32 We have previously shown beneficial effects of six-weeks omega-3 supplementation (1000 mg/day) on hs-CRP levels among women with gestational diabetes mellitus (GDM).33 Likewise, three weeks of supplementation with 200 mg/day vitamin E caused a significant decrease in CRP among patients with coronary heart disease.34 The results of a meta-analysis study have shown that supplementation with vitamin E resulted in decreased levels of CRP.35 However, omega-3 administration for six weeks in subjects with visceral obesity did not cause any significant change in hs-CRP values.36 Co-supplementation with omega-3 and vitamin E for six weeks among patients with GDM showed no significant changes in hs-CRP levels.37 Previous studies reported a 4.4-fold increase in the relative risk for CVD comparing the highest and lowest quartiles of CRP, while this increase is only 2.4-fold comparing the quartiles of cholesterol.38,39 CRP stimulates mononuclear cells to release tissue factors which are important in the initiation of coagulation reactions, complement activation, and neutralization of platelet-activating factor, which in turn promote thrombotic response.39 The beneficial effects of omega-3 and vitamin E co-supplementation on inflammatory cytokines may be due to their effects on decreased production of anti-inflammatory parameters40 and inhibited activation of nuclear factor kappa B.41,42
Our study had some limitations. Due to funding limitations, we did not assess the effects of omega-3 and vitamin E co-supplementation on the measurements of fatty acids profiles and vitamin E. In addition, this study was a relatively short intervention. Long-term interventions might result in better changes in mean levels of CIMT.
Conclusion
Co-supplementation with omega-3 and vitamin E for 12 weeks in patients with PCOS had beneficial effects on CIMT and serum hs-CRP concentration, but unchanged NO values.
Disclosure
The authors declared no conflicts of interest. The study was founded by a grant from the Vice-chancellor for Research, KAUMS, Iran.
references
- 1. Bicer M, Guler A, Kocabas GU, Imamoglu C, Baloglu A, Bilgir O, et al. Endocan is a predictor of increased cardiovascular risk in women with polycystic ovary syndrome. Endocr Res 2017;42(2):145-153.
- 2. Meyer ML, Malek AM, Wild RA, Korytkowski MT, Talbott EO. Carotid artery intima-media thickness in polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update 2012 Mar-Apr;18(2):112-126.
- 3. Luque-Ramírez M, Mendieta-Azcona C, Alvarez-Blasco F, Escobar-Morreale HF. Androgen excess is associated with the increased carotid intima-media thickness observed in young women with polycystic ovary syndrome. Hum Reprod 2007 Dec;22(12):3197-3203.
- 4. Talbott EO, Guzick DS, Sutton-Tyrrell K, McHugh-Pemu KP, Zborowski JV, Remsberg KE, et al. Evidence for association between polycystic ovary syndrome and premature carotid atherosclerosis in middle-aged women. Arterioscler Thromb Vasc Biol 2000 Nov;20(11):2414-2421.
- 5. Orio F Jr, Palomba S, Cascella T, De Simone B, Di Biase S, Russo T, et al. Early impairment of endothelial structure and function in young normal-weight women with polycystic ovary syndrome. J Clin Endocrinol Metab 2004 Sep;89(9):4588-4593.
- 6. Simon A, Gariepy J, Chironi G, Megnien JL, Levenson J. Intima-media thickness: a new tool for diagnosis and treatment of cardiovascular risk. J Hypertens 2002 Feb;20(2):159-169.
- 7. Wang TJ, Nam BH, Wilson PW, Wolf PA, Levy D, Polak JF, et al. Association of C-reactive protein with carotid atherosclerosis in men and women: the Framingham Heart Study. Arterioscler Thromb Vasc Biol 2002 Oct;22(10):1662-1667.
- 8. Saravanan P, Davidson NC, Schmidt EB, Calder PC. Cardiovascular effects of marine omega-3 fatty acids. Lancet 2010 Aug;376(9740):540-550.
- 9. Djoussé L, Folsom AR, Province MA, Hunt SC, Ellison RC; National Heart, Lung, and Blood Institute Family Heart Study. Dietary linolenic acid and carotid atherosclerosis: the National Heart, Lung, and Blood Institute Family Heart Study. Am J Clin Nutr 2003 Apr;77(4):819-825.
- 10. Lonn EM, Bosch J, Diaz R, Lopez-Jaramillo P, Ramachandran A, Hâncu N, et al; GRACE and ORIGIN Investigators. Effect of insulin glargine and n-3FA on carotid intima-media thickness in people with dysglycemia at high risk for cardiovascular events: the glucose reduction and atherosclerosis continuing evaluation study (ORIGIN-GRACE). Diabetes Care 2013 Sep;36(9):2466-2474.
- 11. Stampfer MJ, Hennekens CH, Manson JE, Colditz GA, Rosner B, Willett WC. Vitamin E consumption and the risk of coronary disease in women. N Engl J Med 1993 May;328(20):1444-1449.
- 12. Azen SP, Qian D, Mack WJ, Sevanian A, Selzer RH, Liu CR, et al. Effect of supplementary antioxidant vitamin intake on carotid arterial wall intima-media thickness in a controlled clinical trial of cholesterol lowering. Circulation 1996 Nov;94(10):2369-2372.
- 13. Hodis HN, Mack WJ, LaBree L, Mahrer PR, Sevanian A, Liu CR, et al; VEAPS Research Group. Alpha-tocopherol supplementation in healthy individuals reduces low-density lipoprotein oxidation but not atherosclerosis: the Vitamin E Atherosclerosis Prevention Study (VEAPS). Circulation 2002 Sep;106(12):1453-1459.
- 14. Taghizadeh M, Jamilian M, Mazloomi M, Sanami M, Asemi Z. A randomized-controlled clinical trial investigating the effect of omega-3 fatty acids and vitamin E co-supplementation on markers of insulin metabolism and lipid profiles in gestational diabetes. J Clin Lipidol 2016 Mar-Apr;10(2):386-393.
- 15. Ramezani A, Koohdani F, Djazayeri A, Nematipour E, Keshavarz SA, Saboor Yaraghi AA, et al. Effects of administration of omega-3 fatty acids with or without vitamin E supplementation on adiponectin gene expression in PBMCs and serum adiponectin and adipocyte fatty acid-binding protein levels in male patients with CAD. Anatol J Cardiol 2015;15(12):981-989.
- 16. Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril 2004 Jan;81(1):19-25.
- 17. Tatsch E, Bochi GV, Pereira Rda S, Kober H, Agertt VA, de Campos MM, et al. A simple and inexpensive automated technique for measurement of serum nitrite/nitrate. Clin Biochem 2011 Mar;44(4):348-350.
- 18. Kajbaf MH, Khorvash F, Mortazavi M, Shahidi S, Moeinzadeh F, Farajzadegan Z, et al. Does Omega-3 supplementation decrease carotid intima-media thickening in hemodialysis patients? J Res Pharm Pract 2016 Oct-Dec;5(4):252-256.
- 19. Skilton MR, Ayer JG, Harmer JA, Webb K, Leeder SR, Marks GB, et al. Impaired fetal growth and arterial wall thickening: a randomized trial of ω-3 supplementation. Pediatrics 2012 Mar;129(3):e698-e703.
- 20. Baldassarre D, Amato M, Eligini S, Barbieri SS, Mussoni L, Frigerio B, et al. Effect of n-3 fatty acids on carotid atherosclerosis and haemostasis in patients with combined hyperlipoproteinemia: a double-blind pilot study in primary prevention. Ann Med 2006;38(5):367-375.
- 21. Salonen RM, Nyyssönen K, Kaikkonen J, Porkkala-Sarataho E, Voutilainen S, Rissanen TH, et al; Antioxidant Supplementation in Atherosclerosis Prevention Study. Six-year effect of combined vitamin C and E supplementation on atherosclerotic progression: the Antioxidant Supplementation in Atherosclerosis Prevention (ASAP) Study. Circulation 2003 Feb;107(7):947-953.
- 22. Sacks FM, Stone PH, Gibson CM, Silverman DI, Rosner B, Pasternak RC; HARP Research Group. Controlled trial of fish oil for regression of human coronary atherosclerosis. J Am Coll Cardiol 1995 Jun;25(7):1492-1498.
- 23. Angerer P, Kothny W, Störk S, von Schacky C. Effect of dietary supplementation with omega-3 fatty acids on progression of atherosclerosis in carotid arteries. Cardiovasc Res 2002 Apr;54(1):183-190.
- 24. Hjerkinn EM, Abdelnoor M, Breivik L, Bergengen L, Ellingsen I, Seljeflot I, et al. Effect of diet or very long chain omega-3 fatty acids on progression of atherosclerosis, evaluated by carotid plaques, intima-media thickness and by pulse wave propagation in elderly men with hypercholesterolaemia. Eur J Cardiovasc Prev Rehabil 2006 Jun;13(3):325-333.
- 25. Rizos EC, Ntzani EE, Bika E, Kostapanos MS, Elisaf MS. Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: a systematic review and meta-analysis. JAMA 2012 Sep;308(10):1024-1033.
- 26. Devaraj S, Tang R, Adams-Huet B, Harris A, Seenivasan T, de Lemos JA, et al. Effect of high-dose alpha-tocopherol supplementation on biomarkers of oxidative stress and inflammation and carotid atherosclerosis in patients with coronary artery disease. Am J Clin Nutr 2007 Nov;86(5):1392-1398.
- 27. de Groot PC, Dekkers OM, Romijn JA, Dieben SW, Helmerhorst FM. PCOS, coronary heart disease, stroke and the influence of obesity: a systematic review and meta-analysis. Hum Reprod Update 2011 Jul-Aug;17(4):495-500.
- 28. van der Meer IM, Iglesias del Sol A, Hak AE, Bots ML, Hofman A, Witteman JC. Risk factors for progression of atherosclerosis measured at multiple sites in the arterial tree: the Rotterdam Study. Stroke 2003 Oct;34(10):2374-2379.
- 29. Hurst RT, Ng DW, Kendall C, Khandheria B. Clinical use of carotid intima-media thickness: review of the literature. J Am Soc Echocardiogr 2007 Jul;20(7):907-914.
- 30. De Caterina R. n-3 fatty acids in cardiovascular disease. N Engl J Med 2011 Jun;364(25):2439-2450.
- 31. Rizza S, Tesauro M, Cardillo C, Galli A, Iantorno M, Gigli F, et al. Fish oil supplementation improves endothelial function in normoglycemic offspring of patients with type 2 diabetes. Atherosclerosis 2009 Oct;206(2):569-574.
- 32. Li K, Huang T, Zheng J, Wu K, Li D. Effect of marine-derived n-3 polyunsaturated fatty acids on C-reactive protein, interleukin 6 and tumor necrosis factor α: a meta-analysis. PLoS One 2014 Feb;9(2):e88103.
- 33. Jamilian M, Samimi M, Kolahdooz F, Khalaji F, Razavi M, Asemi Z. Omega-3 fatty acid supplementation affects pregnancy outcomes in gestational diabetes: a randomized, double-blind, placebo-controlled trial. J Matern Fetal Neonatal Med 2016;29(4):669-675.
- 34. Leichtle A, Teupser D, Thiery J. Alpha-tocopherol distribution in lipoproteins and anti-inflammatory effects differ between CHD-patients and healthy subjects. J Am Coll Nutr 2006 Oct;25(5):420-428.
- 35. Saboori S, Shab-Bidar S, Speakman JR, Yousefi Rad E, Djafarian K. Effect of vitamin E supplementation on serum C-reactive protein level: a meta-analysis of randomized controlled trials. Eur J Clin Nutr 2015 Aug;69(8):867-873.
- 36. Chan DC, Watts GF, Barrett PH, Beilin LJ, Mori TA. Effect of atorvastatin and fish oil on plasma high-sensitivity C-reactive protein concentrations in individuals with visceral obesity. Clin Chem 2002 Jun;48(6 Pt 1):877-883.
- 37. Jamilian M, Hashemi Dizaji S, Bahmani F, Taghizadeh M, Memarzadeh MR, Karamali M, et al. A randomized controlled clinical trial investigating the effects of omega-3 fatty acids and vitamin E co-supplementation on biomarkers of oxidative stress, inflammation and pregnancy outcomes in gestational diabetes. Can J Diabetes 2017 Apr;41(2):143-149.
- 38. Danesh J, Wheeler JG, Hirschfield GM, Eda S, Eiriksdottir G, Rumley A, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med 2004 Apr;350(14):1387-1397.
- 39. Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation 2003 Jan;107(3):363-369.
- 40. Hassan Eftekhari M, Aliasghari F, Babaei-Beigi MA, Hasanzadeh J. Effect of conjugated linoleic acid and omega-3 fatty acid supplementation on inflammatory and oxidative stress markers in atherosclerotic patients. ARYA Atheroscler 2013 Nov;9(6):311-318.
- 41. Rossi A, Kapahi P, Natoli G, Takahashi T, Chen Y, Karin M, et al. Anti-inflammatory cyclopentenone prostaglandins are direct inhibitors of IkappaB kinase. Nature 2000 Jan;403(6765):103-108.
- 42. Wu D, Han SN, Meydani M, Meydani SN. Effect of concomitant consumption of fish oil and vitamin E on production of inflammatory cytokines in healthy elderly humans. Ann N Y Acad Sci 2004 Dec;1031:422-424.