Primary choriocarcinoma of the ovary is a rare malignancy and constitutes 0.6% of all ovarian neoplasms.1 Usually, it presents as a component of a mixed germ cell tumor.2 Patients with pure non-gestational choriocarcinoma of the ovary (NGCO) frequently present with abdominal pain and a palpable lower abdominal mass. They occasionally present with metastasis to the lung, pelvis, and vagina. The central nervous system is rarely involved without pulmonary metastasis.3 Primary or metastatic choriocarcinoma of the gastrointestinal (GI) tract is rare.4
We report the case of a young lady who presented with massive bleeding per rectum and was diagnosed with metastatic choriocarcinoma of the ovary to the terminal jejunal segment.
Case Report
A 34-year-old married woman, gravida 3, para 2, and abortion 1, was admitted following 15 days of fever, dry cough, and generalized weakness. The patient noticed progressive weight loss and episodes of melena for five days before admission. Her menstrual cycle was regular, and her last menstrual period was one month before admission. On examination, the patient looked dehydrated and markedly pale with bilateral pitting pedal edema. She was febrile (38.3 °C), tachycardic (130 beats/minute), and normotensive (110/70 mmHg). Except for a palpable mass in the right adnexal region, systemic and gynecological examinations were unremarkable.
Laboratory investigations revealed microcytic hypochromic anemia with hemoglobin of 5.6 g/dL. White cells, platelet counts, and renal and liver functions were within normal limits. A transvaginal ultrasound detected normal sized uterus with prominent right ovary. A chest X-ray showed a large homogeneous density in the right lower lobe of the lung (7 × 6 cm).
During hospitalization, the patient developed massive hematochezia requiring six units of packed red blood cells. An emergency gastroscopy and colonoscopy could not localize the bleeding site. Computed tomography (CT) angiogram revealed extravasation of contrast into the jejunum with associated jejunal mural thickening [Figure 1a]. The patient underwent diagnostic laparoscopy which showed dilated erythematous small bowel loops filled with blood and bulky right ovary with cystic changes. The procedure was converted to an exploratory laparotomy, which revealed nodular thickening (full thickness) of the small bowel extending from the duodenojejunal junction to the proximal part of ileum and multiple enlarged lymph nodes in the entire mesentery. The terminal portion of jejunum and proximal part of ileum was resected with restoration of bowel continuity.
The lumen showed numerous nodules of varying size from 2 mm to 7 mm, some showing ulceration through the mucosa. Intervening mucosa between nodules was unremarkable. There were no mucosal polyps [Figure 1b]. Microscopy revealed numerous submucosal malignant tumor nodules composed of sheets of atypical cytotrophoblastic cells surrounded by syncytial giant cells and large areas of hemorrhage [Figure 1c]. A diagnosis of choriocarcinoma was made.
Further investigations revealed a markedly elevated beta-human chorionic gonadotropin (β-hCG) levels (877 414.0 IU/L). Staging CT scan revealed an irregular lung mass (7.9 × 6.5 × 7 cm) [Figure 1d], two hypervascular right renal lesions, an ill-defined left renal metastatic lesion [Figure 1e], a splenic lesion, a right ovarian enhancing hemorrhagic mass, and several intra-axial metastatic lesions to the brain with peri-focal edema, mass effect, and significant midline shift [Figure 1f].
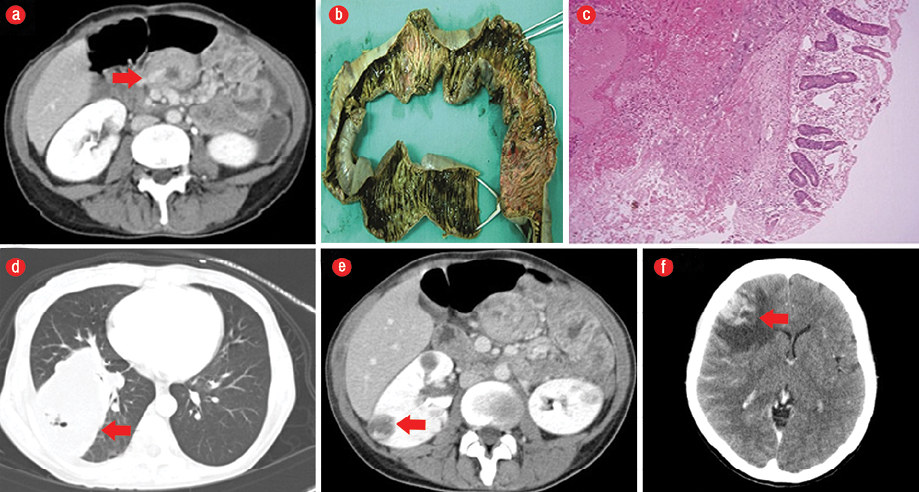
Figure 1: (a) Computed tomography (CT) angiogram revealing bleeding into the jejunal wall and its lumen (red arrow). (b) Macroscopic appearance of the resected specimen revealed multiple hemorrhagic bleeding ulcers with sharp margins in the intestinal mucosa. (c) Hematoxylin and eosin staining revealed malignant cytotrophoblast cells infiltrating the submucosa of the intestine, magnification = 40 ×. (d) CT revealing right basal pulmonary metastatic lesion (red arrow), (e) bilateral renal metastatic lesions (red arrow), and (f) the biggest cerebral metastatic lesion (red arrow).
The patient received dexamethasone and 30 Gy in five fractions. On the fourth day of radiotherapy, the patient presented again with fainting episodes and severe tenderness in the left hypochondrium; her hemoglobin level was 3.5 g/dL. CT scan revealed massive splenic hemorrhage resulting in expansion of splenic capsule along with blood in the perisplenic spaces. An emergency splenic artery embolization was performed, which arrested further bleeding.
The patient was started on combination chemotherapy consisting of bleomycin, etoposide, and cisplatin (BEP). Following two cycles of chemotherapy, we noted a significant decline in β-hCG to 43 064.0 IU/L. Restaging CT scan showed partial remission in the lung, spleen, kidneys, and ovary. The treatment was continued for another four cycles; bleomycin was omitted in the last two cycles. However, because β-hCG was still not within the normal limits, the treatment was modified to vinblastine, ifosfamide, and cisplatin (VeIP). Subsequently, β-hCG normalized [Figure 2]. The patient continued to be in clinical remission six months after the completion of chemotherapy.
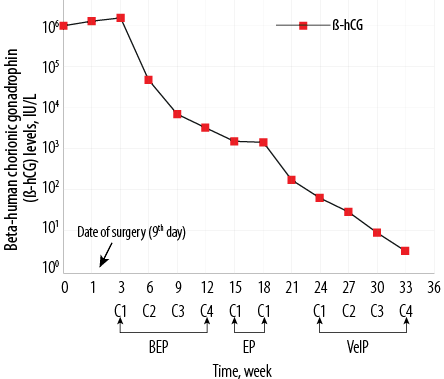 BEP: bleomycin, etoposide, and cisplatin; EP: etoposide and cisplatin;
BEP: bleomycin, etoposide, and cisplatin; EP: etoposide and cisplatin;
VeIP: vinblastine, ifosfamide, and cisplatin.
Figure 2: Serum β-human chorionic gonadotropin levels as a fraction of time on a semi-log scale.
Discussion
NGCO usually affects patients < 20 years of age, however, a few cases have been reported in older patients.5,6 A significant number of patients present with metastatic disease, the lung being the most common site.7 To the best of our knowledge, this is the first case of NGCO with concurrent presentation of the GI tract, spleen, lung, kidney, and central nervous system metastases. A massive GI bleed from an obscured source led to the diagnosis. Metastasis to the small intestine from NGCO is uncommon.4,7,8 Common causes of bleeding from the small intestine are vascular lesions, neoplasms, inflammatory lesions (like Crohn’s disease), Meckel diverticulum, arteriovenous malformation, lymphangiectasia, and radiation-induced injuries. Primary cancers of small intestine presenting with GI bleed are the second most common cause of small intestine bleeding (5–10%). Various malignancies with metastasis to the small intestine present as GI bleeding, which includes GI stromal tumor, intestinal lymphangiomas, Kaposi’s sarcoma, melanoma, and breast cancer.8–10
Metastatic lesions in the small intestine usually present with occult or minimal bleeding. Our patient initially might have been bleeding a minimal amount of blood from her jejunal tumor (as evident by a hypochromic microscopic picture on presentation) but later developed massive bleeding. As the source was the small intestine, upper and lower GI endoscopic examination did not show any bleeding source. A similar case of a young woman with massive GI bleeding has been reported previously.8 Like our patient, she also required surgical intervention and was found to have metastasis to her proximal jejunum from choriocarcinoma. Most patients with NGCO and metastasis to the small gut have simultaneous live metastasis, however, in our case, the liver was spared despite significant metastatic disease to the small intestine, kidneys, and lungs.
Usually, most women with a diagnosis of choriocarcinoma have a preceding history of hydatidiform mole, an abortion, ectopic pregnancy, or full-term pregnancy.8 Previous abortion may be the culprit for the metastatic choriocarcinoma in our case.
Due to the rarity of the disease and lack of randomized studies, the disease is treated by extrapolating the data from studies of germ cell tumors of the testis.11 A combination of BEP has remained the standard of care.12
According to the International Germ Cell Cancer Collaborative Group criteria, our patient had poor risk disease, with very high β-hCG, and non-pulmonary metastases.13 Indeed, the level of β-hCG in excess of one million is one of the highest reported and simultaneous involvement of lungs, kidneys, spleen, and GI tract. The brain has not been reported to the best of our knowledge. Patients with non-metastatic choriocarcinoma have excellent treatment-related outcome and survival rates, but patients with metastatic disease do not do that well.7,10 Taken together the chances of achieving long-term disease-free remission in our patient was < 45%.
The patient was treated with six cycles of BEP, followed by four cycles of VeIP chemotherapy. After four cycles of this combination, the patient achieved serological remission. At the end of treatment, CT scan showed resolution of renal lesions with decreased lung and brain lesions.
Conclusion
Our patient, a 34-year-old woman who presented with massive GI bleeding, had choriocarcinoma of the ovary with multiple metastases and responded to combination chemotherapy. She is now in remission. Metastatic choriocarcinoma is a rare cause of profuse per-rectal bleeding.
Disclosure
The authors declared no conflicts of interest.
references
- 1. Vance RP, Geisinger KR. Pure nongestational choriocarcinoma of the ovary. Report of a case. Cancer 1985 Nov;56(9):2321-2325.
- 2. Goswami D, Sharma K, Zutshi V, Tempe A, Nigam S. Nongestational pure ovarian choriocarcinoma with contralateral teratoma. Gynecol Oncol 2001 Feb;80(2):262-266.
- 3. Sengupta SK, Hamilton DR. Primary nongestational choriocarcinoma of the ovary. Aust N Z J Obstet Gynaecol 1981 Nov;21(4):252-253.
- 4. Iyomasa S, Senda Y, Mizuno K, Miyake H, Amemiya T, Yamaguchi J, et al. Primary choriocarcinoma of the jejunum: report of a case. Surg Today 2003;33(12):948-951.
- 5. Raju GC, Woo J, Marchack D, Naraynsingh V. Primary nongestational choriocarcinoma of the ovary. Postgrad Med J 1985 Aug;61(718):757-758.
- 6. Park SH, Park A, Kim JY, Kwon JH, Koh SB. A case of non-gestational choriocarcinoma arising in the ovary of a postmenopausal woman. J Gynecol Oncol 2009 Sep;20(3):192-194.
- 7. Kumar J, Ilancheran A, Ratnam SS. Pulmonary metastases in gestational trophoblastic disease: a review of 97 cases. Br J Obstet Gynaecol 1988 Jan;95(1):70-74.
- 8. Khawaja FI, Barlas S. Gastrointestinal bleeding from the jejunal metastatic choriocarcinoma: a case report. Saudi J Gastroenterol 1997 Jan;3(1):56-59.
- 9. Pennazio M. Introduction to small-bowel bleeding. Tech Gastrointest Endosc 2012;14(2):94-99.
- 10. Molina Infante J, Beceiro Pedreno I, Ripoll Noiseux C, Marin Jimenez I, Gonzalez Asanza C, Menchen Fernandez-Pacheco P. Gastrointestinal hemorrhage due to metastatic choriocarcinoma with gastric and colonic involvement. Revista espanola de enfermedades digestivas: organo oficial de la Sociedad Espanola de Patologia Digestiva 2004;96(1):77-80.
- 11. Williams S, Blessing JA, Liao SY, Ball H, Hanjani P. Adjuvant therapy of ovarian germ cell tumors with cisplatin, etoposide, and bleomycin: a trial of the Gynecologic Oncology Group. J Clin Oncol 1994 Apr;12(4):701-706.
- 12. Lu KH, Gershenson DM. Update on the management of ovarian germ cell tumors. J Reprod Med 2005 Jun;50(6):417-425.
- 13. International Germ Cell Cancer Collaborative Group. International Germ Cell Consensus Classification: a prognostic factor-based staging system for metastatic germ cell cancers. J Clin Oncol 1997 Feb;15(2):594-603.