Chronic rhinosinusitis (CRS) is defined as inflammation of the nose and the paranasal sinuses. It is characterized by two or more symptoms, one of which should be either nasal blockage/obstruction/congestion or nasal discharge (anterior/posterior nasal drip), and other findings can be facial pain/pressure or reduction/loss of smell and either endoscopic signs of nasal polyps, and/or mucopurulent discharge primarily from middle meatus and/or edema/mucosal obstruction primarily in middle meatus and/or computed tomography changes: mucosal changes within the ostiomeatal complex and/or sinuses that lasted ≥ 12 weeks without complete symptom resolution.1,2 In the last three decades, the prevalence of CRS due to fungal infection has been increasing, especially with the increasing number of immunodeficient patients such as acquired immunodeficiency syndrome, organ transplantation, and cancer therapy.3 Maharani et al,4 found the fungus through polymerase chain reaction (PCR) of the maxillary sinus irrigation fluid of all CRS patients. An estimated 5–15% incidence of CRS is caused by allergic fungal rhinosinusitis, and the prevalence of other classifications of chronic fungal rhinosinusitis is still unclear. Chronic fungal rhinosinusitis is a health concern because it has major impacts on patients’ quality of life, and more difficult medical treatment when compared to CRS due to other causes.2
Not all fungal species can be identified in cases of CRS. Several species are suspected to play a role in CRS such as Alternaria, Aspergillus, Candida, Penicillium, and others. The diversity of species associated with fungal infections in the human body is strongly influenced by geographical conditions, but Candida and Aspergillus are the common species found as pathogens and quite often cause fatality.3 A study conducted by Maharani et al,4 performed on 29 patients with CRS found Aspergillus flavus in 21 subjects and Candida spp. (C. albicans and C. parapsilosis) in 17 subjects. Both Candida spp., Aspergillus spp. and several other fungal species have β-glucan, a polymer of glucose or polysaccharide on the inside of the cell wall that play a role in leukocyte activation. Induction of immune responses through the activation of dectin-1 by recognizing β-glucan is an important foundation for the response of the adaptive immune system and will lead to the recruitment of large amounts of neutrophils to help eliminate fungi in tissue and will increase neutrophil/eosinophil ratio (NER). However, this is not always the case, in circumstances where the fungus acts as an antigen there will be T-helper 2 (Th2) inflammatory activation leading to eosinophil cell recruitment (decreased NER).5–7
β-glucan levels of bronchoalveolar lavage (BAL) and blood serum are known to increase in 87.5% of patients with Pneumocystis pneumonia.5 This indicates the role of β-glucan as a diagnostic marker for fungal infection and is expected to reduce invasive procedure, especially when 38–86% of invasive fungal infections reveal negative microscopic and culture tests result, indicating the difficulty of diagnosis.7 Invasive fungal rhinosinusitis from Aspergillus spp. can be detected by utilizing the antigen of Aspergillus galactomannan and using the enzyme-linked immunosorbent assay (ELISA) technique, which has a sensitivity of 64% and a specificity of 60%.8 If β-glucan is shown to be applicable as a diagnostic test, it will be advantageous since it covers more fungal species than the Aspergillus galactomannan antigen examination. Histopathologic examination of the number of dominant inflammatory cells (besides fungal morphology) is also needed to help guide the diagnosis. By knowing the dominant inflammatory cell, it can give a rough idea of the underlying pathophysiology of a disease (whether it is allergy or infection).9 It is useful in helping doctors determine the appropriate treatment for the patient. However, so far, no literature supports this especially those that correlate β-glucan with NER.
β-glucan play roles in the host immune system, particularly as an immunomodulator with its specific receptor, dectin-1. However, due to the incomplete understanding about the pathophysiology of CRS and the lack of β-glucan-related or NER-related studies, especially the possibility of utilization as a marker of CRS, the investigators are interested in further investigation. This research could provide additional knowledge, facilitate the diagnosis and therapy of chronic fungal rhinosinusitis in the future, and provide basic data for further research.
Methods
We conducted observational analytic research using a cross-sectional approach to determine the correlation between β-glucan and the NER of paranasal sinus mucosa and blood in the diagnosis of chronic fungal rhinosinusitis in Saiful Anwar General Hospital, Malang. The study was approved by the Saiful Anwar General Hospital Ethical Committee and written informed consent was obtained from all patients. Patients did not receive any stipend from the researcher, but all examination fees are borne by the researcher. The research was done between September 2016 and September 2017. Based on the calculation, there are 20 samples. We used the consecutive sampling technique.
Patients with chronic maxillary rhinosinusitis with or without nasal polyps aged ≥ 18 years old during the study, underwent maxillary sinus surgery and had positive PCR results for fungi with β-glucan were included in the study. Patients were excluded if they received antifungal (systemic or topical) treatment for ≥ 4 weeks, or systemic corticosteroids for ≥ 7 days with equivalent doses of 40 mg/day methylprednisolone or topical corticosteroid for ≥ 1 month with equivalent dose of fluticasone propionate 400 µg/day.
As part of a blood test before undergoing maxillary sinus surgery, 5 mL of blood was taken for β-glucan ELISA and neutrophil and eosinophil flow cytometry and stored in two vacutainer tubes with EDTA. The patient then underwent surgery with anterior antrostomy (canine fossa puncture or Caldwell Luc procedure) with general or local anesthesia conducted by the rhinology division of the Otorhinolaryngology Head and Neck Surgery Department at Saiful Anwar General Hospital. A specimen of maxillary sinus mucosa was collected in a sterile container, and during transport, the specimen was stored in a cooling box for PCR, β-glucan ELISA, and neutrophil and eosinophil flow cytometry.
Patients were included if PCR examination revealed fungi with β-glucan, including Aspergillus flavus, Aspergillus fumigatus, Candida albicans, Candida parapsilosis, and Cryptococcus neoformans. PCR examination used the Jena Bioscience® DNA Preparation Kit, Intron Maxime® PCR Premix (master mix), DNA marker, and IDT® DNA primer. PCR, ELISA, and flow cytometry examination were performed. Patients who did not meet the inclusion criteria were excluded from the analysis and were still treated according to the applicable clinical practice guidelines.
The ELISA examination used the Finetest® Human β-glucan ELISA Kit. Levels of β-glucan serum blood and paranasal sinus mucosa examinations 60 pg/mL were considered negative, intermediate at 60–79 pg/mL, and positive if ≥ 80 pg/mL. The flow cytometry examination used BioLegend® Neutrophil and Eosinophil Kit Flow Cytometry. Levels of eosinophil (blood) were normal when 3–9% of total cells and for paranasal sinus mucosa is 0.3–0.7% of total cells. Levels of neutrophil in blood were normal in the range of 55.8–59.6%, and 1.1–1.7% of total cells for paranasal sinus mucosa. The NER was calculated using the following formula:
Ratio = Neutrophil
Eosonophil
The NER threshold value in paranasal sinus mucosa is 2.8, while for blood is 9.6. NER above the limit indicate the presence of neutrophilic inflammation and NER below the limit indicates eosinophilic inflammation.
All data obtained was processed using SPSS Statistics (IBM Corp. Released 2011. IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp). The suitability between elevated β-glucan levels of sinus mucosa with blood serum and NER of sinus mucosa with blood serum were analyzed by paired t-test/Wilcoxon (alternative). The correlation between β-glucan and NER of sinus mucosa and blood were analyzed using the Pearson/Spearman test (alternative). A statistically significant decrease was considered if p < 0.050.
Results
In the period of study, 24 patients with chronic maxillary rhinosinusitis with or without nasal polyp underwent maxillary sinus surgery. After PCR examination, 20 patients with chronic maxillary fungal rhinosinusitis fulfilled the inclusion and exclusion criteria. Only a few samples in this study showed specific characteristics of fungal infection in paranasal sinuses such as a fungal ball [Figure 1]. The diagnosis of fungal rhinosinusitis cannot be establish by the clinical characteristic alone but need additional culture or pathological examination. From five identified species, Aspergillus flavus was the most common species found in this study (n = 14), followed by Aspergillus fumigatus (n = 13), Candida albicans (n = 10), Cryptococcus neoformans (n = 3), and Candida parapsilosis (n = 2).
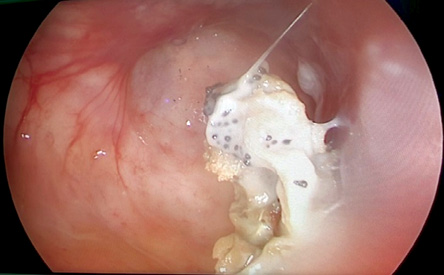
Figure 1: One study subject with fungal ball (polymerase chain reaction and culture test positive for Aspergillus fumigatus).
Table 1: The levels and suitability of β-glucan maxillary sinus mucosa and blood serum.
|
Mucosa, pg/mL |
20 |
944.8 ± 981.8 |
622.0 (179–4551) |
< 0.001 |
0.735 |
0.886 |
*after data transformation; SD: standard deviation.
Table 2: Suitability of neutrophil/eosinophil ratio (NER) of maxillary sinus mucosa and blood.
|
Mucosa |
20 |
2.0 ± 2.4 |
1.3 (0.2–9.1) |
< 0.001 |
0.153 |
< 0.001 |
*after data transformation; SD: standard deviation.
β-glucan as a wall component of several fungal species can be utilized in the diagnosis of chronic fungal rhinosinusitis. In this research, the mean maxillary sinus mucosa β-glucan level was 944.8±981.8 pg/mL, whereas in blood this was 909.6±487.3 pg/mL [Table 1]. Paired t-tests were performed to determine the suitability between mucosa and blood β-glucan. There was no significant difference between β-glucan mucosal level and blood (p = 0.886), indicating that blood β-glucan level can represent the β-glucan level in the paranasal sinus mucosa. A patient who meets the CRS criteria with positive blood β-glucan can be considered as having chronic fungal rhinosinusitis, although future research is needed. Based on the positive β-glucan positive limit, all subjects crossed the threshold for mucosal specimens, and only one subject with blood-β-glucan levels corresponded to the intermediate limit.
The end-product of dectin-1 stimulation by β-glucan is neutrophil cell recruitment. Flow cytometry was done against blood specimens and maxillary sinus mucosa. The mean neutrophil count (%) in mucosa was 4.3±2.4%, and eosinophil count (%) was 4.0±2.9%. The mean neutrophil count in blood was 50.9±11.8%, and eosinophil count was 4.4±2.0%. Based on these data we then performed calculations to determine the NER and establish the dominant inflammatory response. The most common inflammatory response in the maxillary sinus mucosa was eosinophilic inflammation (n = 17) whereas in blood it was neutrophilic inflammation (n = 12). Paired t-tests were performed to determine the suitability between mucosa and blood NER. There was a significant difference between mucosal NER with blood (p < 0.001) indicating that the inflammatory response of blood did not represent the inflammatory response in paranasal sinus mucosa [Table 2]. Based on the results of Pearson correlation test, β-glucan level with NER in both maxillary sinus mucosa and blood had no significant correlation (p > 0.050), meaning that elevation of β-glucan did not cause NER changes [Table 3].
Table 3: Correlation between β-glucan and neutrophil/eosinophil ratio (NER) of paranasal sinus mucosa and blood.
|
Mucosal β-glucan with
mucosal NER |
0.618 |
-0.119 |
Discussion
CRS is a high prevalence disease. There are several guidelines to help establish the diagnosis, one of which is European Position Paper on Rhinosinusitis and Nasal Polyps. These guidelines combine subjective complaints with clinical findings from both physical and laboratory examination.1,2
If patients with CRS there treated according to the algorithm showed no improvement, it should be suspected that the cause is a fungal infection but an invasive procedure is required to prove the diagnosis. Other alternative examinations are often insensitive or expensive. A simpler examination, such as IgE Aspergillus, is still difficult to apply in Indonesia and some regions in the world due to the lack of facilities. It is therefore necessary to have a new modality that is cheaper and easier to perform but minimally invasive, especially with the increasing incidence of fungal infections (including fungal rhinosinusitis) in the last three decades.1,5
Fungal spores are small and easily carried by the wind, therefore, it can be found anywhere and inhaled by humans resulting in colonization of the nasal and paranasal sinuses mucosa. Not all fungal species cause CRS because the fungus is opportunistic. Some species that cause abnormalities in humans have cell walls composed by β-glucan. The most widely reported species were Aspergillus fumigatus, Aspergillus flavus, and Candida albicans.7,10 In this study, Aspergillus flavus was the most common species, found in 70.0% of subjects. The differences of most common species found between this study and the literature (mostly from India) can be caused by different geographical factors, humidity, and climate. Indonesia has a tropical climate with high humidity, when compared to India, which has a tropical and subtropical climate with low humidity. In this study, direct sampling of the maxillary sinus was obtained through anterior antrostomy to avoid contamination of the nasal cavity, and the sample was stored in a sterile container. Fungal identification is an important but difficult process because of the limitations of available examination. Although expensive and difficult to do, PCR has the highest sensitivity and specificity compared to other tests used to identify fungi.11,12
Together with chitin, β-glucan provides strength and a framework for some fungal species and constitutes 60.0% of the fungal cell wall components, comprising 40.0% β-1,3-glucan and 20.0% β-1,6-glucan. Glucan is located at position 1,3 and 1,6 on the glucose chain and can distinguish glucan belonging to fungi with the property of wheat or similar crops, where β-glucan can be found in 1,3 and 1,4 [Figure 2]. In this research, we used a specific fungal β-glucan reagent with 1,3 and 1,6 glucose branch to prevent the influence of other substances that may raise blood β-glucan level and confused the results. β-glucan is a target for the immune response, but it does not always appear in all parts of the fungus. β-glucan will be expressed primarily on conidia (an infected asexual fungal spore), and before it is expressed, β-glucan will hide on the deep fungal cell wall and be difficult to recognize by the host immune system. In this study, there was no significant difference between β-glucan mean values on paranasal sinus mucosa and blood serum. This suggests that β-glucan may be used as a marker of fungal infection in the paranasal sinus mucosa, but further research is needed for diagnostic testing by comparing outcomes with healthy people or those with CRS due to other causes.5,6,13,14
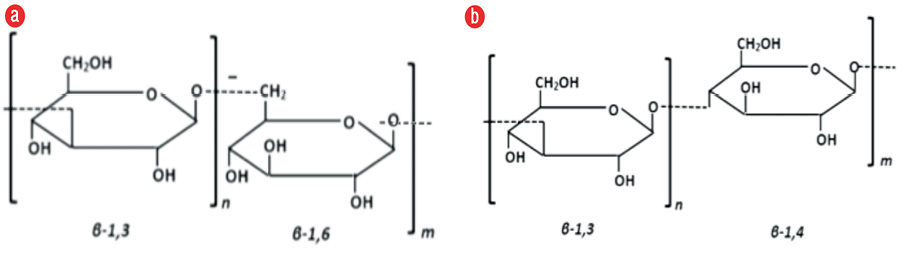
Figure 2: Comparison of β-glucan on the glucose chain: (a) fungal β-glucan and (b) crop β-glucan.
To date, there have been no other studies related to levels of β-glucan on the paranasal sinus mucosa. A similar study obtained β-glucan levels from BAL and blood samples of Pneumocystis pneumonia patients with a median value of 500 pg/mL (BAL) and 406 pg/mL (blood).5 The results were higher than in the control group, which obtained a median value of 50 pg/mL (BAL) and 42 pg/mL (blood). All subjects passed the positive β-glucan threshold, except for one who had an intermediate value on blood specimens. These values have now been used by the United States Food and Drug Agency as the limit value for the diagnosis of fungal infections. It is also currently being investigated for the possibility of evaluating β-glucan levels for decision making to give antifungal treatment in suspected cases of fungemia with unknown location of infection.15–18
Stimulation of the immune system by β-glucan has occurred since the introduction of β-glucan by dectin-1, which is a specific β-glucan receptor. The presence of fungi in the paranasal sinus mucosa can damage the unity of mechanical barriers with the release of proteolytic enzymes. The body will try to eliminate pathogens by deploying various immune system effectors to the site of infection. The response of the human immune system to eliminate β-glucan is by enzyme production, is a nonspecific process, although the β-glucan introduction process involves a specific receptor. The human body’s attempt to exclude β-glucan is by the oxidative degradation process of active oxygen and nitric ions produced by macrophage or leukocyte cells, but the details of the process are still not fully understood. The process runs slowly and is considered ineffective in removing the fungus, which causes the fungus component to be identified within the body for long periods of time. Monitoring β-glucan levels can be used to assess the efficacy of fungal infection therapy with regular monitoring. If antifungal treatment is successful, there should be a decrease in β-glucan levels. In addition, although β-glucan is the largest component of fungal cell walls, its inner location is sometimes unrecognizable by the host immune system. These conditions allow the fungus to colonize and penetrate the vascular endothelium where it is carried away by the systemic bloodstream without activating the immune system. In these circumstances, the fungi component including β-glucan can be found in the blood circulation, and it can be used as a marker of fungal infections, especially invasive fungal infections.3,19–21
The use of β-glucan as a marker of fungal infections has been presented in some literature, but the study has not been done for cases of fungal rhinosinusitis. Pazos et al,22 obtained a sensitivity of 88%, specificity of 90%, positive predictive value of 70%, and negative predictive value of 96% for β-glucan as a marker of fungal infection. A similar study using galactomannan antigen as a marker of fungal infections with ELISA examination found a sensitivity of 64% and a specificity of 60%.8 The weakness of the galactomannan ELISA examination is that it can only detect infections caused by Aspergillus spp. when compared with β-glucan, which covers more fungal species. Ongoing research using the combination of β-glucan examination with galactomannan is expected to provide better diagnostic capability covering more fungal species. Both examinations are also being developed to assess the success of antifungal treatment by evaluating β-glucan levels at the start and during the course of treatment.18,21,23
Exposure of the fungus to the paranasal sinus mucosa will further stimulate the host immune system causing T-cell polarization. When dectin-1 is activated, there will be naive T-cell polarization to Th1 or Th17, while if the fungus infiltrates the host body as an antigen, then naive T-cells will differentiate to Th2 cells.1 No research has been done to evaluate the prevalence of dominant inflammatory cells in people with fungal rhinosinusitis, especially in Indonesia, but allergic fungal rhinosinusitis as the most common form of fungal rhinosinusitis has a dominance of eosinophil cells.2,24 Differences in stimulants may cause different inflammatory responses even if they are caused by the same pathogens, in which case the fungus may stimulate the immune system as microorganisms or, as in this study, the fungus tends to act as an antigen. In this study, there was a significant difference between NER on paranasal sinus mucosa and blood. This suggests that NER cannot be used as a supporting marker for β-glucan in the diagnosis of fungal infection, especially because there is no correlation between β-glucan and NER.
The difference in the type of inflammation between mucosa (eosinophilic) and blood (neutrophilic) may be due to the process of in situ hematopoiesis in which inflammatory progenitor cells differentiate (or become active) once they infiltrate the site of infection. Assessment of inflammation that occurs should not only focus on effector cells, but also proinflammatory cytokines because although there is no correlation between paranasal sinus mucosa inflammatory cells and blood cytokines involved such as interleukin (IL)-5 or gamma interferon (IFN-g), which can increase both in tissue and in blood, still need to be evaluated.25,26
Neutrophils are the largest leukocyte component in the human body and are the first line of defense against fungi. The inflammatory responses in the case of fungal rhinosinusitis are initiated by the interaction between pathogens and the immune system, either by antigen presenting cells or neutrophils. The introduction of these pathogens will then stimulate the production of proinflammatory cytokines and cause T-cell differentiation to Th1/Th17 or Th2. With the activation of dectin-1, there will be an increase in inflammatory cell recruitment, which can be seen with an increase in the number of neutrophils (and an increase of the NER) and the amount of dectin-1 expressed on the surface of neutrophil cells at the site of infection. In this condition, the physician may need to consider antifungal treatment as an adjuvant of surgical treatment. The decrease of circulatory neutrophil cells can be attributed to inadequate production due to insufficient stimulants to stimulate the production of neutrophils.27–30
Many studies, including this study, indicate that fungal infections are more related to eosinophilic inflammation, especially in cases of fungal rhinosinusitis with nasal polyps. Th2 cytokines such as IL-5, IL-13, and regulated on activation normal T-cell expressed and secreted (RANTES) stimulate the production, survival, and recruitment of eosinophils to the site of inflammation. The white blood cell component is produced in the bone marrow and circulates in the blood with the stimulation of proinflammatory cytokines, which are recruited to the site of inflammation. The inflammatory response is a systemic inflammatory reaction that works locally and is characterized by an increase in the number of effector cells in tissue that are not always accompanied by an increase in effector cells in the blood circulation, especially in chronic conditions. This can be seen by evaluating eosinophil cationic proteins, an eosinophil activity marker that increases in the tissues, but not in the blood.25,28,31–33
β-glucan is the largest constituent of the cell wall of the fungus, but it does not mean the human immune system can easily recognize it. β-glucan fungi consist of two chains, namely 1,3 and 1,6. The 1,3 chain has a greater proportion than 1,6, but the 1,6 chain bond is stronger and effective in stimulating neutrophils to induce phagocytosis and reactive oxygen species production.33 However, neutrophil stimulation would not occur if it did not activate dectin-1. The role of dectin-1 as a bridge in the protective immune system against fungal infection is further emphasized in this study, where the presence of β-glucan in large quantities alone is not sufficient without the introduction of dectin-1 to cause adequate neutrophil recruitment.6,34 There are several mechanisms for the fungus to escape the immune system, including shielding of proinflammatory pathogen-associated molecular patterns, modulation of the inflammatory response (as opposed to protection response), releasing the bait to outwit the immune system, avoid phagocytosis response, persistent confrontation, and avoiding the complement system.19,35,36
Previous studies have not specifically shown a correlation between β-glucan levels and changes in the NER, but focus on the response of one effector cell, so there is no comparative data between the results of this study and other studies. Several fungal species, including Aspergillus spp. and Candida spp. stimulate the production of IL-5, IL-13, and RANTES, which are Th2 cytokines. In these circumstances, the fungus acts as an antigen and carries superoxide dismutase that creates an environment rich in Th2 cytokines. This study shows that changes in the NER toward neutrophil dominance requires not only β-glucan in large quantities, but its effects in stimulating dectin-1.37 Unfortunately, our study results indicate a diagnosis of fungal infections acquired with β-glucan as a marker, cannot be sharpened by knowing the underlying pathophysiology, whether infection or allergies, because there is no correlation between blood and mucosal NER. Diagnosis can only be achieved by blood tests, and still need an invasive procedure to determine appropriate treatment. It is known that rhinosinusitis can be caused by fungal infection requiring surgery without waiting for maximum medical treatment, but adjuvant steroid or antifungal treatment must await surgical findings.
Conclusion
The presence of β-glucan in the blood can be used as a marker of chronic fungal rhinosinusitis by keeping in mind the presence of symptoms and physical finding that match with diagnosis criteria of CRS. However, differential diagnosis of allergies and infections cannot be excluded by examination of blood NER. Still, it is hoped that with this research the process of diagnosing chronic fungal rhinosinusitis can be obtained quickly and precisely without the need for an invasive procedure so that appropriate treatment can be immediately provided. Further research is needed for diagnostic test and proof of β-glucan linkage with the pathophysiology of fungal rhinosinusitis.
Disclosure
The authors declared no conflict of interest. This research received funding from a Saiful Anwar General Hospital research grant (grant no. 070/270/1.20/302/2017), which was used for the procurement of all reagents and funds that were personally provided by the author.
Acknowledgements
We would like to thank Umi Salamah from Physiology Laboratory Medical Faculty of Brawijaya University who assisted us in processing all specimens and Nanik Setijowati, M.D. from Public Health Department Medical Faculty of Brawijaya University who helped in statistical analysis.
references
- 1. Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, et al. European position paper on rhinosinusitis and nasal polyps 2012. Rhinol Suppl 2012 Mar;23:3, 1-298.
- 2. Adelson RT, Marple BF, Ryan MW. Fungal rhinosinusitis. In: Johnson JT, Rosen CA, editors. Bailey’s head and neck surgery-otolaryngology review. 5th ed. Baltimore: Lippincott Williams & Wilkins; 2014. p. 557-572.
- 3. Wüthrich M, Deepe GS Jr, Klein B. Adaptive immunity to fungi. Annu Rev Immunol 2012;30:115-148.
- 4. Maharani I, Suheryanto R, Retnoningsih E. Airborne Fungi in Chronic Rhinosinusitis Patients Maxillary Sinus Lavage at Dr. Saiful Anwar Hospital Malang. Bali Medical Journal. 2016;5(2):18-24.
- 5. Theel ES, Jespersen DJ, Iqbal S, Bestrom JE, Rollins LO, Misner LJ, et al. Detection of (1, 3)-b-D-glucan in bronchoalveolar lavage and serum samples collected from immunocompromised hosts. Mycopathologia 2013 Feb;175(1-2):33-41.
- 6. Akramienė D, Kondrotas A, Didžiapetrienė J, Kėvelaitis E. Effects of beta-glucans on the immune system. Medicina (Kaunas) 2007;43(8):597-606.
- 7. Shetty A, Rodrigues C. Microbiology in invasive fungal sinusitis. In: Mankekar G, editor. Invasive fungal rhinosinusitis. New Delhi: Springer; 2014. p. 39-50.
- 8. Chen CY, Sheng WH, Cheng A, Chen YC, Tsay W, Tang JL, et al. Invasive fungal sinusitis in patients with hematological malignancy: 15 years experience in a single university hospital in Taiwan. BMC Infect Dis 2011 Sep;11(250):250.
- 9. Meltzer EO, Hamilos DL. Rhinosinusitis diagnosis and management for the clinician: a synopsis of recent consensus guidelines. Mayo Clin Proc 2011 May;86(5):427-443.
- 10. Brewer JM, Marple BF. Fungal Disease in the Maxillary Sinus. In: Duncavage JA, Becker SS, editors. The maxillary sinus - medical and surgical management. New York: Thieme Medical Publishers, Inc; 2011. p. 50-59.
- 11. Hebart H, Loeffler J, Einsele H. Molecular Diagnostics: Present and Future. In: Maertens JA, Marr KA, editors. Diagnosis of Fungal Infections. New York: Informa Healthcare USA, Inc; 2007. p. 133-152.
- 12. Guarner J, Brandt ME. Histopathologic diagnosis of fungal infections in the 21st century. Clin Microbiol Rev 2011 Apr;24(2):247-280.
- 13. Champagne JP, Antisdel JL, Woodard TD, Kountakis SE. Epidemiologic factors affect surgical outcomes in allergic fungal sinusitis. Laryngoscope 2010 Nov;120(11):2322-2324.
- 14. Hohl TM, Van Epps HL, Rivera A, Morgan LA, Chen PL, Feldmesser M, et al. Aspergillus fumigatus triggers inflammatory responses by stage-specific beta-glucan display. PLoS Pathog 2005 Nov;1(3):e30.
- 15. Odabasi Z, Mattiuzzi G, Estey E, Kantarjian H, Saeki F, Ridge RJ, et al. Beta-D-glucan as a diagnostic adjunct for invasive fungal infections: validation, cutoff development, and performance in patients with acute myelogenous leukemia and myelodysplastic syndrome. Clin Infect Dis 2004 Jul;39(2):199-205.
- 16. Deshazo RD. Syndromes of invasive fungal sinusitis. Med Mycol 2009;47(1)(Suppl 1):S309-S314.
- 17. Albert O, Toubas D, Strady C, Cousson J, Delmas C, Vernet V, et al. Reactivity of (1→3)-b-d-glucan assay in bacterial bloodstream infections. Eur J Clin Microbiol Infect Dis 2011 Nov;30(11):1453-1460.
- 18. De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, et al; Revised Definitions of Invasive Fungal Disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 2008;46(12):1813-1821.
- 19. Gow NA, van de Veerdonk FL, Brown AJ, Netea MG. Candida albicans morphogenesis and host defence: discriminating invasion from colonization. Nat Rev Microbiol 2011 Dec;10(2):112-122.
- 20. Kern RC, Conley DB, Walsh W, Chandra R, Kato A, Tripathi-Peters A, et al. Perspectives on the etiology of chronic rhinosinusitis: an immune barrier hypothesis. Am J Rhinol 2008 Nov-Dec;22(6):549-559.
- 21. Miura NN. Fate of β-glucans In Vivo: Organ distribution and degradation mechanisms of fungal β-glucans in the body. In: Young SH, Castranova V, editors. Toxicology of 1 -3-Beta-glucans: glucans as a marker for fungal exposure. Boca Raton: Taylor & Francis; 2005. p. 109-126.
- 22. Pazos C, Ponton J, Del Palacio A. Contribution od (1-3)-β-D-glucan chromogenic assay to diagnosis and therapeutic monitoring of invasive aspergillosis in neutropenic adult patients: a comparison with serial screening for circulating galactomannan. J Clin Microbiol 2005 Jan;43(1):299-305.
- 23. Morrison CJ, Warnock DW. Serodiagnosis: antibody and antigen detection. In: Maertens JA, Marr KA, editors. Diagnosis of fungal infections. New York: Informa Healthcare; 2007. p. 65-120.
- 24. Gong J, Wang P, Qiu ZH, Chen QJ. Increased expression of dectin-1 in nasal polyps. Am J Otolaryngol 2013 May-Jun;34(3):183-187.
- 25. Denburg JA, Keith PK. Systemic aspects of chronic rhinosinusitis. Immunol Allergy Clin North Am 2004 Feb;24(1):87-102.
- 26. Denburg JA, Sehmi R, Saito H, Pil-Seob J, Inman MD, O’Byrne PM. Systemic aspects of allergic disease: bone marrow responses. J Allergy Clin Immunol 2000 Nov;106(5)(Suppl):S242-S246.
- 27. Taylor PR, Tsoni SV, Willment JA, Dennehy KM, Rosas M, Findon H, et al. Dectin-1 is required for beta-glucan recognition and control of fungal infection. Nat Immunol 2007 Jan;8(1):31-38.
- 28. Fidel PL Jr. Candida albicans: from commensal to pathogen. In: Tannock GW, editor. Medical importance of the normal microflora. Dunedin: Springer; 1999. p. 441-476.
- 29. Ozment-Skelton TR, deFluiter EA, Ha T, Li C, Graves BM, Ferguson DA Jr, et al. Leukocyte Dectin-1 expression is differentially regulated in fungal versus polymicrobial sepsis. Crit Care Med 2009 Mar;37(3):1038-1045.
- 30. Kennedy AD, Willment JA, Dorward DW, Williams DL, Brown GD, DeLeo FR. Dectin-1 promotes fungicidal activity of human neutrophils. Eur J Immunol 2007 Feb;37(2):467-478.
- 31. Feger TA, Rupp NT, Kuhn FA, Ford JL, Dolen WK. Local and systemic eosinophil activation in allergic fungal sinusitis. Ann Allergy Asthma Immunol 1997 Sep;79(3):221-225.
- 32. Romani L. Cell mediated immunity to fungi: a reassessment. Med Mycol 2008 Sep;46(6):515-529.
- 33. Pant H, Hughes A, Miljkovic D, Schembri M, Wormald P, Macardle P, et al. Accumulation of effector memory CD8+ T cells in nasal polyps. Am J Rhinol Allergy 2013 Sep-Oct;27(5):e117-e126.
- 34. Rubin-Bejerano I, Abeijon C, Magnelli P, Grisafi P, Fink GR. Phagocytosis by human neutrophils is stimulated by a unique fungal cell wall component. Cell Host Microbe 2007 Jul;2(1):55-67.
- 35. Chai LY, Netea MG, Vonk AG, Kullberg BJ. Fungal strategies for overcoming host innate immune response. Med Mycol 2009 May;47(3):227-236.
- 36. da Silva Dantas A, Lee KK, Raziunaite I, Schaefer K, Wagener J, Yadav B, et al. Cell biology of Candida albicans-host interactions. Curr Opin Microbiol 2016 Dec;34:111-118.
- 37. Okano M, Fujiwara T, Haruna T, Kariya S, Makihara S, Higaki T, et al. Role of fungal antigens in eosinophilia-associated cellular responses in nasal polyps: a comparison with enterotoxin. Clin Exp Allergy 2011 Feb;41(2):171-178.