There are an estimated 148 million Muslim with diabetes worldwide.1 Most of them fast during the holy month of Ramadan. During this fasting period, Muslims abstain from food and drink (including oral medications) from dawn to dusk. The duration of fasting can reach up to 19 hours in some parts of the world, especially during summertime. Fasting during Ramadan is a cardinal religious obligation for those who can withstand fasting. The effects of fasting during Ramadan for those with diabetes have been studied over the last two decades. The aims of clinical studies in this field are to determine who can fast safely and which diabetes medicines can be used safely during Ramadan.
The Epidemiology of Diabetes and Ramadan (EPIDIAR) and CREED studies, as well as others, have highlighted the most-encountered risks of fasting in patients with diabetes: diabetic ketoacidosis (DKA), hyperglycemia, dehydration, hypoglycemia, and thrombosis.2–4 Dehydration is a greater concern in hot climates especially the Middle East, which has the vast majority of Muslims.
Many clinical trials have assessed the safety of various pharmacological diabetes treatments during Ramadan,5–7 including insulin, sulphonylureas (SUs), glucagon-like peptide-1 (GLP-1) agonists, and dipeptidyl peptidase inhibitors (DPP-IV).5–14 Based on the evidence from these trials, many guidelines and recommendations did not show much concern to the use of most of these agents during Ramadan except SUs and intensive insulin regimens.15–17
Over the last few years, the use of the relatively new sodium-glucose cotransporter 2 inhibitors (SGLT2-I) has grown, particularly after the favorable cardiovascular and renal effects of empagliflozin.18 These SGLT2-I inhibit glucose reabsorption in the proximal renal tubules, where 90% of glucose reabsorption occurs.19 The resulting osmotic diuresis carries a higher risk of hypovolemia as well as higher rates of urinary tract infections. Furthermore, the Food and Drug Administration published a warning after some reports of DKA and acute kidney injury following the use of SGLT2-I.20
Hazards of SGLT2-I are expected to be higher with prolonged fasting, but to date, only two studies have assessed use of SGLT2-I during Ramadan.21,22 In both studies, SGLT-2-I showed lower rates of hypovolemia and hypoglycemia during fasting compared to SUs. Though DKA and renal compromise are reported outside Ramadan, they have not been reported during Ramadan.
Our primary objective was to assess the safety of SGLT2-I during Ramadan, in a real-life scenario, by finding the frequency and severity of hypoglycemic events as well as the frequency of dehydration. The secondary objective was to determine the effect of SGLT2-I on weight, glycated hemoglobin (HbA1c), lipid profile, and creatinine during Ramadan.
Methods
This prospective trial was performed at two Dubai Health Authority sites. The trial protocol was reviewed and approved by the institutional review board and ethics committee of the DHA. Informed consent was obtained from participants in the study.
All Muslim patients more than 18 years old with type 2 diabetes treated with SGLT2-I (canagliflozin 100 mg or dapagliflozin 10 mg) on 30 March 2016 were screened for eligibility for the trial. All patients had an estimated glomerular filtration rate (eGFR) ≥ 60 mL/min/m2 (as per hospital protocols for prescription of an SGLT2-I). We excluded data of patients who discontinued the SGLT2-I before or during Ramadan and those who fasted for less than 20 days. As part of the study protocol, we conducted a phone interview, those who could not be reached by phone were excluded.
Most patients attended Ramadan-focused education sessions either formally or during physician consultations as part of routine practice in our hospital. We reviewed the electronic database as well as patients’ medical records for demographic and biological data, detailed history of diabetes, antidiabetic medications, comorbidities, and laboratory results performed within a month before Ramadan. These included HbA1c, lipid profile, and serum creatinine. Laboratory tests performed within six weeks after Ramadan were also collected. All information was transcribed onto a data collection sheet. Accuracy was ensured by another investigator.
Within six weeks after Ramadan, patients were interviewed by telephone using a structured questionnaire that included nine questions [Box 1].
Box 1: Standardized telephone questionnaire questions conducted within six weeks of Ramadan.
- Did you have symptoms of hypoglycemia?
|
- If yes, did you check your blood sugar?
|
- If yes, did this confirm the hypoglycemia?
|
- What time did you develop hypoglycemia?
|
- If it was during fasting, did you break your fast?
|
- Did you change your insulin or oral hypoglycemic agent dose during Ramadan?
|
- Did you have symptoms of severe dehydration (extreme and unusual thirst)?
|
- Have you ever been admitted due to high or low blood sugar level or extreme dehydration?
|
- How many days did you fast during Ramadan?
|
A patient was considered to have type 2 diabetes if the American Diabetes Association criteria for the diagnosis of diabetes mellitus was fulfilled (fasting blood glucose (FBG) ≥ 126 mg/dL, random blood glucose (RBG) ≥ 200 mg/dL, or HbA1c ≥ 6.5%) or if an antidiabetic agent was taken as a treatment. Patients on metformin alone were not considered diabetic unless the blood levels were met.
We defined hypoglycemia as blood glucose < 70 mg/dL. Severe hypoglycemia was considered if the patient required third-party assistance or admission to the emergency room.
The overall rate of hypoglycemia and differences in occurrence rates was assessed based on the concomitant treatment used along with SGLT2-I. Hence, we looked at the rate of hypoglycemia in patients using SGLT2-I with insulin and those using SGLT2-I with other oral hypoglycemic agents (OHA). We further compared those on SGLT2-I plus intensive insulin therapy versus those on SGLT2-I plus basal insulin only and those on SGLT2-I plus SUs versus those on SGLT2-I plus other oral agents (not including SUs).
Data analysis was performed using SPSS Statistics (SPSS Inc. Released 2007. SPSS for Windows, Version 16.0. Chicago, SPSS Inc.), and significance was recognized when p < 0.050. Quantitative variables were described using the mean, standard deviation (SD), and range, whereas qualitative variables were defined using values and percentages.
The chi-square test was used to compare groups using qualitative variables. The unpaired t-test was used to compare groups using quantitative variables and in parametric data (SD < 50% of the mean). The Mann-Whitney test was used instead of the t-test when the data were non-parametric. The paired t-test was used to compare the same group before and after treatment using parametric data, and the Wilcoxon test if the data were non-parametric.
Results
A total of 535 patients being treated with one of the two SGLT2-I by March 30, 2016, were screened for inclusion in the study. Of these, 71 were excluded because the telephone interview was not completed. Another 30 were excluded for not fasting during Ramadan. A further six were excluded because treatment was stopped before Ramadan, either because it was unavailable or because of the fear of experiencing hypoglycemia during Ramadan. An additional 10 were excluded because of a lack of baseline data in the electronic database. Finally, one patient who died as a result of a traffic accident was excluded. The total number of patients included was 417.
The included patients were primarily female (58.5%), aged 54.0±11.6 years, and weighed 83.8±16.6 kg. Their mean HbA1c level was 8.3±1.7% and the mean duration of diabetes was 13.4±6.6 years (range three months to 37 years). The medications used at baseline are given in Table 1.
Hypoglycemia symptoms were seen in 113 patients (27.0%). Of these, 93 (82.3%) checked their blood glucose levels and 78 (83.8%) confirmed their hypoglycemia (blood glucose of < 70 mg/dL). Despite the advice to break fasting in case of hypoglycemia, only 38 patients broke their fast. The total number of patients who adjusted their insulin or OHA during Ramadan was 57 (13.6%). One (0.2%) patient was admitted to the hospital with symptoms of hypoglycemia. Hypoglycemic events occurred mostly in the evening (67 episodes, 64.4%), but also in the afternoon (25 episodes, 24.0%), morning, and after breaking fast (16 episodes, 15.4% each).
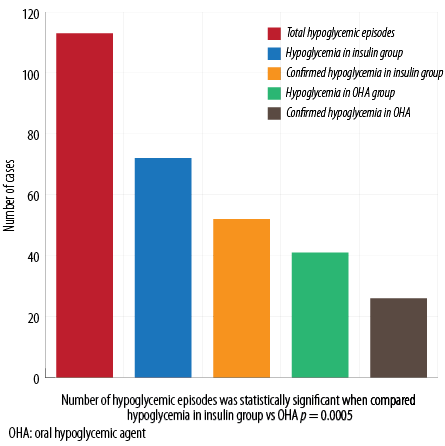
Figure 1: Total and confirmed hypoglycemic episodes in insulin and OHA groups.
Table 1: Baseline characteristics of participants.
|
Females |
58.5 (n = 244) |
|
Age, years, mean ± SD |
54.0 ± 11.6 |
|
Duration of diabetes, years, mean ± SD |
13.4 ± 6.6 |
|
Weight, kg, mean ± SD |
83.8 ± 16.6 |
|
Medicine |
|
|
Insulin |
45.8 (n = 191) |
|
Metformin |
89.9 (n = 375) |
|
DPP-4 inhibitors |
52.7 (n = 220) |
|
GLP-1 analogues |
39.0 (n = 163) |
|
Sulphonylureas |
46.0 (n = 192) |
|
TZDs |
8.6 (n = 36) |
|
Laboratories |
|
|
HbA1c, mean ± SD |
8.3 ± 1.7 |
DPP-4: dipeptidyl peptidase-4; GLP-1: glucagon-like peptide-1;
TZDs: thiazolidinediones; SD: standard deviation; . HbA1c: glycated hemoglobin
Total number of patients on SGLT2-I and OHA (SGLT2-I+OHA) was 227, while those on SGLT2-I and insulin (SGLT2-I+insulin) was 190. There was a statistically significant difference in the number of hypoglycemic episodes when we compared patients using SGLT2-I+insulin versus those using SGLT2-I and OHA (72 episodes (37.8%) vs. 41 episodes (18.0%), p < 0.005). This difference was observed despite the fact that more patients taking SGLT2-I+insulin adjusted their doses during Ramadan compared to those using SGLT2-I+OHA (44 patients (23.15%) vs. 13 patients (5.7%), respectively, p < 0.001). No difference between the timing of hypoglycemia was found in either group [Figure 1].
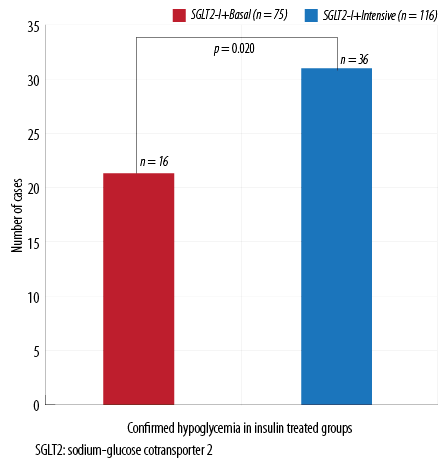
Figure 2: Confirmed hypoglycemia in insulin subgroups.
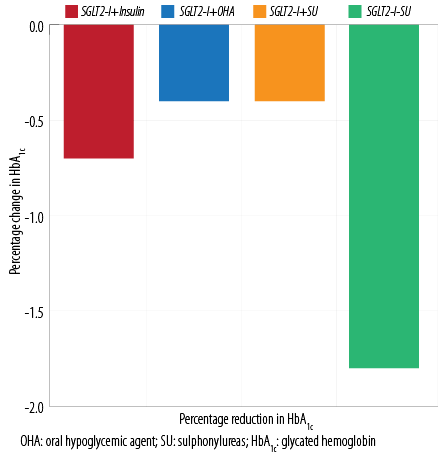
Figure 3: Change in HbA1c in the groups.
No difference in the occurrence of confirmed hypoglycemic events was found between patients on SGLT2-I plus SUs compared to patients using SGLT2-I without SUs (n = 24 out of 132 patients on SUs (18.1%) vs. n = 18 out of 94 patients on other OHA (18.5%), p = 0.770). Similarly, no difference in the percentage of overall hypoglycemic episodes was found between the two insulin subgroups (basal vs. multiple daily injections (MDI)). However, confirmed hypoglycemic episodes were more significant in the intensive insulin therapy group (p = 0.020; Figure 2).
Moreover, significantly more patients broke their fast in the intensive insulin therapy group, n = 23 out of 116 patients on MDI (19.8%) vs. n = 5 out of 74 of patients on basal insulin (6.7%) in the SGLT2-I plus basal insulin (p = 0.004).
Only 39 (9.3%) patients cohort complained of extreme and unusual thirst. These symptoms were seen more frequently among patients treated with SGLT2-I plus insulin than those treated with SGLT2-I plus OHAs (n = 25 (13.15%) in the insulin-treated group vs. n = 14 (6.16%) in the OHA treated group, respectively, p = 0.030).
We recorded no cases of DKA, thrombotic events, or changes in serum creatinine. The mean HbA1c level was 8.3±1.7% before Ramadan and significantly reduced to 7.8±1.3% (p < 0.001) after Ramadan. At baseline, HbA1c was significantly higher in the SGLT2-I plus insulin-treated group than in those treated with SGLT2-I plus OHAs (8.9±1.7% vs. 7.8±1.5% respectively, p < 0.001). By the end of Ramadan, a significant reduction was noted in both groups (8.2±1.5% vs. 7.4±1.4% respectively, p < 0.001) [Figure 3].
Changes in mean HbA1c remained significant after correction for age, sex, duration of diabetes, and comorbidities. Mean weight at baseline was 83.9±17.0 kg, which reduced to 83.8±16.6 kg after Ramadan (p < 0.001). Changes in mean weight remained significant after correction for age, sex, duration of diabetes, and comorbidities.
Discussion
The safety and efficacy of SGLT2-I as add-on therapies to metformin and other OHAs, as well as other intensive insulin therapy, has been established in several randomized controlled trials.23–26 Among the notable beneficial effects of this class of OHAs is its promising cardiovascular and renal outcomes.18,27 Concerns center around its osmotic diuretic effect, which can lead to dehydration, an increased frequency of genital infections, and, less commonly, euglycemic ketoacidosis.28,29
These concerns come to the forefront when the use of SGLT2-I during Ramadan is considered, particularly in hot climates where the combination of long hours of fasting and warm weather could potentially increase the risks of dehydration, thrombosis, and hypoglycemia. Ramadan fasting in diabetic patients also poses risks of hyperglycemia and DKA due to several factors, among which are eating habits that are altered to include more carbohydrates, late-night eating, and elimination or adjustment of diabetes medications.2,3 The latest recommendation for the management of diabetes during Ramadan suggested that the use of SGLT2-I during Ramadan might be an attractive option given the low risk of hypoglycemia. However, the risk of volume contraction in elderly patients raises some concern. The authors suggested that more randomized controlled trials are required before being certain about their safety.30
A recent survey of physicians’ views on the use of SGLT2-I in Ramadan reported that the majority (70.6%) of 197 physicians surveyed believed that SGLT2-I were generally appropriate and safe to use during Ramadan but should be discontinued in a select group of patients, while 16.2% believed they were safe to use in all patients despite the lack of safety evidence. The authors advised that SGLT2-I should not be prescribed immediately before Ramadan.31
Our study differed from the two other available studies that assessed the safety and tolerability of SGLT2-I during Ramadan in that it evaluated, in a real-life scenario, this class of antidiabetic drugs on top on ongoing treatment that included insulin. The other two studies evaluated SGLT2-I on top of metformin with or without DPP-4 inhibitor compared to SUs.21,22 The first was a randomized, 12-week, open-label, two-arm parallel group study using 110 patients. It assessed the hypoglycemia risk and safety of dapagliflozin compared with SU as an addition to metformin during Ramadan. The incidence of hypoglycemia was lower with dapagliflozin (6.9% vs. 28.8%, p = 0.002). No difference in the incidence of urinary tract infections or postural hypotension was found between the groups.21 The second study was a non-randomized prospective parallel cohort that assessed use of canagliflozin compared with SUs. Fewer patients in the canagliflozin group experienced hypoglycemia (adjusted odd ratio (OR) 0.273; 95% confidence interval (CI): 0.0104–0.719).22
In our cohort, the higher risk hypoglycemia was noted in patients using insulin, which has been shown to increase the likelihood of developing hypoglycemia during fasting and non-fasting periods.6,32–35
The frequency of hypoglycemia was not statistically significant when we compared patients on SGLT2-I on SUs to those on SGLT2-I using other OHAs. This indicates that the use of gliclazide, which is the SU used in more than 95% of our patients, is not associated with increased risk of hypoglycemia. Similar conclusions were derived in another study that included 1021 patients randomized to receive sitagliptin or to remain on a preexisting SU, demonstrated a significantly greater incidence of hypoglycemia among those treated with SUs (6.7% vs. 13.2%, p < 0.001).36 However, when they assessed the SUs associated with hypoglycemia they found that gliclazide was not associated with increased hypoglycemia risk.
It is unfortunate that only a minority of our patients with symptomatic hypoglycemia broke fasting (33.6%) despite the fact that most confirmed hypoglycemia using a glucometer. More education and awareness is needed about fasting with diabetes so that potentially serious hypoglycemia can be avoided.37
Of all patients in the cohort, 9.3% complained of extreme thirst. This is comparable to the 10% incidence of postural hypotension seen in another study.21 The Canagliflozin in Ramadan Tolerance Observational Study (CRATOS) assessed the volume depletion events prospectively in a similar questionnaire.22 The number of volume depletion events was higher in the canagliflozin group compared to the SU group (16.1% reported ≥ 1 volume depletion event in canagliflozin group compared with eight patients 5.0% treated with a SU).22
It is difficult to assess whether thirst was related to SGLT2-I use or other confounding factors such as the nature of their work, level of hydration during non-fasting hours, and intake of high-salt foods.
Consistent with results seen with other SGLT2-I, injectable, and other OHAs studied during Ramadan, all patients on SGLT2-I had a marked reduction in HbA1c levels post-Ramadan; 8.3±1.7% before fasting to 7.8±1.3% (p < 0.001) after Ramadan.
Additionally, significant weight reductions were seen in all groups on SGLT2-I post-Ramadan. In the CRATOS trial, there were a numerically more patients in the canagliflozin group who lost weight (weight change from baseline was -2.4 kg compared to -0.5 kg in the SU group), but this was not statistically significant.22
This was not a randomized trial and certain confounding factors, such as dietary habits, fluid intake, and exercise were not accounted for, possibly affecting the rates of hypoglycemia or dehydration. The use of fructosamine would have been a better measure of short-term glycemic control specifically during Ramadan. However, this study gives a realistic snapshot of the daily practice of adding SGLT2-I to preexisting diabetes treatment regimens, including insulin and SUs. It also provides reliable data on glycemic control and weight changes.
Conclusion
Use of SGLT2-I is safe during fasting by diabetic patients, and they provide a significant reduction in HbA1c and weight post-Ramadan regardless of the underlying diabetes medication. Hypoglycemic episodes are more frequent in patients on insulin, specifically basal bolus or premixed insulins, and adjustment of insulin doses is recommended. A more structured education is needed to improve patient management of hypoglycemic episodes while fasting.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
Acknowledgements
The authors thank the Dubai Health Authority ethical committee for their ongoing support of researchers in Dubai. We also thank Mr. Fahad and Mrs. Badriya from the medical records section of Dubai Hospital for their ongoing support and help in providing medical charts in a timely and organized manner.
references
- 1. Abdul Jabbar. Epidemiology of diabetes and Ramadan fasting [cited 2017 November 8]; p. 17-26. Available from: http://www.daralliance.org/daralliance/wp-content/uploads/IDF-DAR-Practical-Guidelines_15-April-2016_low_2.pdf.
- 2. Salti I, Bénard E, Detournay B, Bianchi-Biscay M, Le Brigand C, Voinet C, et al; EPIDIAR study group. A population-based study of diabetes and its characteristics during the fasting month of Ramadan in 13 countries: results of the epidemiology of diabetes and Ramadan 1422/2001 (EPIDIAR) study. Diabetes Care 2004 Oct;27(10):2306-2311.
- 3. Babineaux SM, Toaima D, Boye KS, Zagar A, Tahbaz A, Jabbar A, et al. Multi-country retrospective observational study of the management and outcomes of patients with type 2 diabetes during Ramadan in 2010 (CREED). Diabet Med 2015 Jun;32(6):819-828.
- 5. Abdelgadir EI, Hassanein MM, Bashier AM, Abdelaziz S, Baki S, Chadli A, et al. A prospective multi-country observational trial to compare the incidences of diabetic ketoacidosis in the month of Ramadan, the preceding month, and the following month (DKAR international). J Diabetes Metab Disord 2016 Nov;15:50
- Gray LJ, Dales J, Brady EM, Khunti K, Hanif W, Davies MJ. Safety and effectiveness of non-insulin glucose-lowering agents in the treatment of people with type 2 diabetes who observe Ramadan: a systematic review and meta-analysis. Diabetes Obes Metab 2015 Jul;17(7):639-648.
- 6. Aravind SR, Al Tayeb K, Ismail SB, Shehadeh N, Kaddaha G, Liu R, et al; 2009 Ramadan Study Group. Hypoglycaemia in sulphonylurea-treated subjects with type 2 diabetes undergoing Ramadan fasting: a five-country observational study. Curr Med Res Opin 2011 Jun;27(6):1237-1242.
- 7. Brady EM, Davies MJ, Gray LJ, Saeed MA, Smith D, Hanif W, et al. A randomized controlled trial comparing the GLP-1 receptor agonist liraglutide to a sulphonylurea as add on to metformin in patients with established type 2 diabetes during Ramadan: the Treat 4 Ramadan Trial. Diabetes Obes Metab 2014 Jun;16(6):527-536.
- 8. Azar ST, Echtay A, Wan Bebakar WM, Al Araj S, Berrah A, Omar M, et al. Efficacy and safety of liraglutide compared to sulphonylurea during Ramadan in patients with type 2 diabetes (LIRA-Ramadan): a randomized trial. Diabetes Obes Metab 2016 Oct;18(10):1025-1033.
- 9. Khalifa AA, El Rashid AO, Bashier AM. Safety and efficacy of liraglutide as an add-on therapy to pre-existing anti-diabetic regimens during Ramadan, a prospective observational trial. J Diabetes Metab Disord 2015;6:590.
- 10. Pan C, Yang W, Barona JP, Wang Y, Niggli M, Mohideen P, et al. Comparison of vildagliptin and acarbose monotherapy in patients with type 2 diabetes: a 24-week, double-blind, randomized trial. Diabet Med 2008 Apr;25(4):435-441.
- 11. Devendra D, Gohel B, Bravis V, Hui E, Salih S, Mehar S, et al. Vildagliptin therapy and hypoglycaemia in Muslim type 2 diabetes patients during Ramadan. Int J Clin Pract 2009 Oct;63(10):1446-1450.
- 12. Bashir MI, Pathan MF, Raza SA, Ahmad J, Khan AK, Ishtiaq O, et al. Role of oral hypoglycemic agents in the management of type 2 diabetes mellitus during Ramadan. Indian J Endocrinol Metab 2012 Jul;16(4):503-507.
- 13. Hassanein M, Abdallah K, Schweizer A. A double-blind, randomized trial, including frequent patient-physician contacts and Ramadan-focused advice, assessing vildagliptin and gliclazide in patients with type 2 diabetes fasting during Ramadan: the STEADFAST study. Vasc Health Risk Manag 2014 May;10:319-326.
- 14. Halimi S, Levy M, Huet D, Quéré S, Dejager S. Experience with vildagliptin in type 2 diabetic patients fasting during Ramadan in France: Insights from the VERDI study. Diabetes Ther 2013 Dec;4(2):385-398.
- 15. Pathan MF, Sahay RK, Zargar AH, Raza SA, Khan AK, Ganie MA, et al. South Asian Consensus Guideline: Use of insulin in diabetes during Ramadan. Indian J Endocrinol Metab 2012 Jul;16(4):499-502.
- 16. Al-Arouj M, Assaad-Khalil S, Buse J, Fahdil I, Fahmy M, Hafez S, et al. Recommendations for management of diabetes during Ramadan: update 2010. Diabetes Care 2010 Aug;33(8):1895-1902.
- 17. Almalki MH, Alshahrani F. Options for controlling type 2 diabetes during Ramadan. Front Endocrinol (Lausanne) 2016;18;7:32.
- 18. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al; EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015 Nov;373(22):2117-2128.
- 19. Scheen AJ. Pharmacodynamics, efficacy and safety of sodium-glucose co-transporter type 2 (SGLT2) inhibitors for the treatment of type 2 diabetes mellitus. Drugs 2015 Jan;75(1):33-59.
- 20. Sodium-glucose cotransporter-2 (SGLT2) inhibitors. Administration USFD. 2016 [cited 2017 May]. Available from: https://www.fda.gov/Drugs/DrugSafety/ucm446852.htm.
- 21. Wan Seman WJ, Kori N, Rajoo S, Othman H, Mohd Noor N, Wahab NA, et al. Switching from sulphonylurea to a sodium-glucose cotransporter2 inhibitor in the fasting month of Ramadan is associated with a reduction in hypoglycaemia. Diabetes Obes Metab 2016 Jun;18(6):628-632.
- 22. Hassanein M, Echtay A, Hassoun A, Alarouj M, Afandi B, Poladian R, et al. Tolerability of canagliflozin in patients with type 2 diabetes mellitus fasting during Ramadan: Results of the Canagliflozin in Ramadan tolerance observational study (CRATOS). Int J Clin Pract 2017 Oct;71(10).
- 23. Wilding JP, Woo V, Rohwedder K, Sugg J, Parikh S; Dapagliflozin 006 Study Group. Dapagliflozin in patients with type 2 diabetes receiving high doses of insulin: efficacy and safety over 2 years. Diabetes Obes Metab 2014 Feb;16(2):124-136.
- 24. Bailey CJ. Morales Villegas Ec, Woo V, Tang W, Ptaszynska A, List JF. Efficacy and safety of dapagliflozin monotherapy in people with Type 2 diabetes: a randomized double-blind placebo-controlled 102-week trial. Diabet Med 2015;32(4):531-541.
- 25. John M, Cerdas S, Violante R, Deerochanawong C, Hassanein M, Slee A, et al. Efficacy and safety of canagliflozin in patients with type 2 diabetes mellitus living in hot climates. Int J Clin Pract 2016 Sep;70(9):775-785.
- 26. Nauck MA, Del Prato S, Durán-García S, Rohwedder K, Langkilde AM, Sugg J, et al. Durability of glycaemic efficacy over 2 years with dapagliflozin versus glipizide as add-on therapies in patients whose type 2 diabetes mellitus is inadequately controlled with metformin. Diabetes Obes Metab 2014 Nov;16(11):1111-1120.
- 27. Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, et al; EMPA-REG OUTCOME Investigators. Empagliflozin and Progression of kidney disease in type 2 diabetes. N Engl J Med 2016 Jul;375(4):323-334.
- 28. Johnsson K, Johnsson E, Mansfield TA, Yavin Y, Ptaszynska A, Parikh SJ. Osmotic diuresis with SGLT2 inhibition: analysis of events related to volume reduction in dapagliflozin clinical trials. Postgrad Med 2016 May;128(4):346-355.
- 29. Peters AL, Buschur EO, Buse JB, Cohan P, Diner JC, Hirsch IB. Euglycemic diabetic ketoacidosis: A potential complication of treatment with sodium-glucose cotransporter 2 inhibition. Diabetes Care 2015 Sep;38(9):1687-1693.
- 30. Ibrahim M, Abu Al Magd M, Annabi FA, Assaad-Khalil S, Ba-Essa EM, Fahdil I, et al. Recommendations for management of diabetes during Ramadan: update 2015. BMJ Open Diabetes Res Care 2015 Jun;3(1):e000108.
- 31. Beshyah SA, Chatterjee S, Davies MJ. Use of SGLT2 inhibitors during Ramadan: A survey of physicians’ views and practical guidance. The British Journal of Diabetes 2016;16:20-24.
- 32. Cesur M, Corapcioglu D, Gursoy A, Gonen S, Ozduman M, Emral R, et al. A comparison of glycemic effects of glimepiride, repaglinide, and insulin glargine in type 2 diabetes mellitus during Ramadan fasting. Diabetes Res Clin Pract 2007 Feb;75(2):141-147.
- 33. Salti I; Diabetes and Ramadan Study Group. Efficacy and safety of insulin glargine and glimepiride in subjects with type 2 diabetes before, during and after the period of fasting in Ramadan. Diabet Med 2009 Dec;26(12):1255-1261.
- 34. Mattoo V, Milicevic Z, Malone JK, Schwarzenhofer M, Ekangaki A, Levitt LK, et al; Ramadan Study Group. A comparison of insulin lispro Mix25 and human insulin 30/70 in the treatment of type 2 diabetes during Ramadan. Diabetes Res Clin Pract 2003 Feb;59(2):137-143.
- 35. Akram J, De Verga V; Ramadan Study Group. Insulin lispro (Lys(B28), Pro(B29) in the treatment of diabetes during the fasting month of Ramadan. Diabet Med 1999 Oct;16(10):861-866.
- 36. Al Sifri S, Basiounny A, Echtay A, Al Omari M, Harman-Boehm I, Kaddaha G, et al; 2010 Ramadan Study Group. The incidence of hypoglycaemia in Muslim patients with type 2 diabetes treated with sitagliptin or a sulphonylurea during Ramadan: a randomised trial. Int J Clin Pract 2011 Nov;65(11):1132-1140.
- 37. Bravis V, Hui E, Salih S, Mehar S, Hassanein M, Devendra D. Ramadan education and awareness in diabetes (READ) programme for Muslims with type 2 diabetes who fast during Ramadan. Diabet Med 2010 Mar;27(3):327-331.