Neisseria meningitidis is a gram-negative diplococcus exclusively infecting humans. The acute systemic meningococcal disease manifests variously as meningitis, meningitis with meningococcemia, or meningococcemia without clinical evidence of meningitis.1,2
However, unlike in other bacterial meningitis, the focal neurologic signs and seizures are less common in meningococcal meningitis as correlated with postmortem findings.3,4 The reported neurological complications include cerebral infarction, cerebral herniation, hydrocephalus, cranial nerve palsies, and vascular complications.1,3,5−7
Cerebral venous sinus thrombosis (CVST) is an uncommon complication of meningococcal meningitis.1 In contrast, in pneumococcal meningitis the incidence of CVST was reported at 10.3%.6 We present the case of a 42-year-old man diagnosed with CVST associated with meningococcal meningitis and discuss the use of anticoagulant therapy.
Case report
A 42-year-old male, manual laborer with no significant past medical illness was admitted to the emergency department with a four-hour history of altered sensorium, generalized fatigue, headache, and an episode of urinary incontinence. There was no history of associated involuntary movements or symptoms associated with cranial nerve involvement. There was no reliable person available to give details of his past medical illness including the history in the run-up to his current presentation.
On examination, no skin rash was observed. He was stuporous with occasional spontaneous eye opening. He had neck stiffness and could localize to painful stimuli. No other lateralizing signs or papilledema were present on comprehensive neurological examination. The patient was intubated under sedation for respiratory protection. Blood work revealed an elevated white blood cell (WBC) count of 23.43 × 109/L (normal range = 4.00−11.00 × 109/L) with 88% neutrophils and thrombocytopenia with a platelet count of 53 × 109/L (normal range = 140−400 × 109/L). His random blood glucose level was 11.8 mmol/L (normal range = 2.8−8.8 mmol/L), and he had an elevated activated partial thromboplastin time (aPTT) of 41.3 s (normal range = 28.6−38.2 s), and elevated C-reactive protein (CRP) levels of 302.88 mg/L (normal range = 0−5 mg/L). Plain computed tomography (CT) [Figure 1] and CT venogram [Figure 2] were suggestive of straight sinus thrombosis. There were no subdural collections, hydrocephalus, or midline shift.
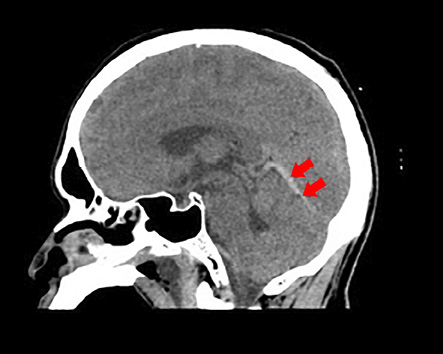
Figure 1: Noncontrast computed tomography of the brain revealed curvilinear hyperdensity (red arrows) along the course of straight sinus.
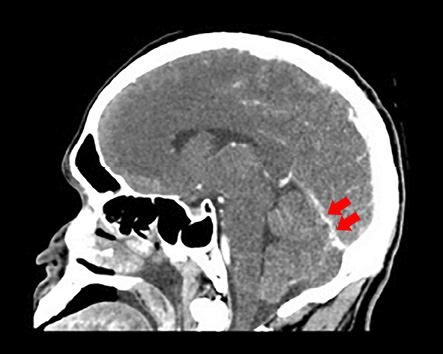
Figure 2: Computed tomography of the brain with contrast revealed a straight sinus with irregular caliber (red arrows).
Empiric therapy with acyclovir, vancomycin, and ceftriaxone was started. Lumbar puncture showed findings suggestive of bacterial meningitis (WBC count of 7631 × 106/L (normal range = 0−5 × 106/L), red blood cell count of 4000 × 106/L, protein level of 5 968 mg/L (range = 150−450 mg/L), and glucose of 0.42 mmol/L (normal range = 2.22−3.89 mmol/L)). The culture of cerebrospinal fluid revealed gram-negative diplococcus, which was confirmed to be N. meningitidis (A/Y antigen by latex agglutination assay). Other blood work showed elevated levels of fibrinogen at 11.2 g/L (normal range = 1.9−4.3 g/L), D-dimer of 1.1 µg/mL (normal ≤ 0.5 µg/mL), blood creatinine levels of 171 µmol/L (normal range = 50−104 µmol/L), urea of 9.7 mmol/L (normal ≤ 8.3 mmol/L), and total and direct bilirubin levels of 22.3 µmol/L (normal ≤ 21 µmol/L) and 19.1 µmol/L (normal ≤ 5 µmol/L), respectively. Total protein and albumin were both decreased at 56 g/L (normal range = 64−83 g/L) and 25 g/L (normal range = 35−52 g/L), respectively.
On the third day, sedation was stopped, and he was extubated. His platelet counts improved to 163 000/µL on the fourth day of his admission. He complained about generalized pressure like headache, which was initially 7/10 severity and decreased to 2−3/10 over the first 10 days. He did not have any neck stiffness after extubation and the headache was treated symptomatically. Over the course of the following week, the patient’s condition continued to improve and ceftriaxone was given for 14 days. Except for a headache, the patient’s course was uneventful and he made a full recovery with improvement in his platelet levels to 202 × 109/L, WBC counts of 12.54 × 109, aPTT of 36 s, and CRP 86.3 mg/L at the end of his antibiotic regimen. At follow-up, one week after discharge, he did not have any symptoms or signs of neurological disease. He was subsequently lost to follow-up.
Discussion
Various infections of central nervous system (CNS) can cause different forms of cerebrovascular disease. In a study of 83 patients with meningitis, cerebrovascular complications were reported in 13 patients.8 The pathological processes involved are either para-infectious immune-mediated causing vasospasm or thrombosis, vasculitis affecting primarily the vessels at the base of the brain in meningitis, or a hypercoagulable state in combination with endothelial dysfunction
resulting from activation of inflammatory and procoagulant cascades.
CNS infections leading to CVST had a wide range of incidence as per literature review. In a multinational, multicenter study, CNS infections accounted for only 2.1% of the 624 patients with CVST.7 In a 2016 single-center prospective cohort study from India, the reported incidence was 19.8% of 87 cases.9 There are reports of cerebral venous thrombosis occurring in association with meningitides caused by Streptococcus pneumonia, Mycobacterium tuberculosis, coccidioidomycosis, and Fusobacterium necrophorum.8,10,11
Meningococcal infection of CNS causing CVST is uncommon.1,6 Disseminated intravascular coagulopathy and vascular thrombosis are known to occur in meningococcal infection due to the high levels of circulating procoagulant microparticles from platelets or granulocytes and disordered protein C activation in endothelial cells.3,12 Meningococcal infection causes increased vascular permeability associated with pathological vasoconstriction, vasodilation and intravascular thrombosis resulting in manifestations such as purpura fulminans to even infarction and gangrene of extremities. Involvement of plasminogen activator inhibitor-1 is suggested as a cause of the thrombotic events.13
The manifestations of invasive meningococcal disease are meningitis, septicemia, or a combination of both. It may also present as pneumonia, conjunctivitis, otitis media, epiglottitis, arthritis, urethritis and pericarditis, chronic meningococcemia, post-meningococcal reactive disease, and self-mutilating behavior.1,2 Headache is by far the most frequent presenting symptom in a patient with CVST.14,15 In a study of 59 patients with CVST, impaired consciousness was reported in 39% of patients.16 Similar findings were also described in a series of 111 patients with CVST where headache and altered sensorium were found to be the presenting feature in 69% and 27% of patients, respectively.15 This was also the case in our patient.
Beneficial effects of heparin in CVST have been reported even in patients with intracerebral hemorrhage. Current consensus guidelines including European Federation of Neurological Societies guidelines 2011, American Academy of Chest Physicians 2012, and American Heart Association/American Stroke Association 2014 support the use of anticoagulation for the acute treatment of CVST. The fifth edition 2016 National Institute for Health and Care Excellence guidelines for stroke recommends that patients with cerebral venous thrombosis (including those with secondary cerebral hemorrhage) should receive a full-dose of anticoagulation therapy (initially a full-dose heparin and then warfarin with a target international normalized ratio of 2–3) for at least three months unless there are comorbidities that preclude their use.
There is considerable debate regarding the use of anticoagulant therapy in the presence of meningococcal infection. Nürnberger et al,17 recommended the use of anticoagulant therapy with or without plasma substitution amongst pediatric patients with systemic meningococcal infection.However, controlled clinical trials with heparin as an adjunctive therapy in meningococcemia have not established its safety or usefulness.18,19 There are case reports wherein anticoagulant therapy was used in the management of CVST in the presence of meningococcal infection.5
Studies done to evaluate the prognosis of CVST showed impaired consciousness, coma, paresis, intracerebral hemorrhages, older age, focal neurological deficits, and septic CVST as poor prognostic factors.14,16,20 Evaluation of these prognostic factors may help in guiding the management of CVST in the presence of complicating conditions such as meningococcal infection on whether or not to adopt anticoagulant measures.
Conclusion
We presented a case of meningococcal meningitis with thrombosis of the straight sinus. The patient was managed with antibiotic therapy and made a complete recovery. The use of anticoagulation in CVST secondary to meningococcal infection needs further studies to establish its usefulness.
Disclosure
The authors declared no conflicts of interest.
references
- 1. Heckenberg SG, de Gans J, Brouwer MC, Weisfelt M, Piet JR, Spanjaard L, et al. Clinical features, outcome, and meningococcal genotype in 258 adults with meningococcal meningitis: a prospective cohort study. Medicine (Baltimore) 2008 Jul;87(4):185-192.
- 2. Dinkar A, Singh J, Atam V, Sahani KK, Patel ML. Self Mutilating Behaviour in Severe Meningococcal Infection; An Interesting Association. J Clin Diagn Res 2016 May;10(5):OD03-OD04.
- Pathan N, Faust SN, Levin M. Pathophysiology of meningococcal meningitis and septicaemia. Arch Dis Child 2003 Jul;88(7):601-607.
- 4. Rockholt M, Cervera C. Images in clinical medicine. Hypoglossal nerve palsy during meningococcal meningitis. N Engl J Med 2014 Oct;371(15):e22.
- 5. Bozzola E, Bozzola M, Colafati GS, Calcaterra V, Vittucci A, Luciani M, et al. Multiple cerebral sinus thromboses complicating meningococcal meningitis: a pediatric case report. BMC Pediatr 2014 Jun;14(1):147.
- 6. Kastenbauer S, Pfister HW. Pneumococcal meningitis in adults: spectrum of complications and prognostic factors in a series of 87 cases. Brain 2003 May;126(Pt 5):1015-1025.
- 7. Ferro JM, Canhão P, Stam J, Bousser MG, Barinagarrementeria F; ISCVT Investigators. Prognosis of cerebral vein and dural sinus thrombosis: results of the International Study on Cerebral Vein and Dural Sinus Thrombosis (ISCVT). Stroke 2004 Mar;35(3):664-670.
- 8. Pfister HW, Feiden W, Einhäupl KM. Spectrum of complications during bacterial meningitis in adults. Results of a prospective clinical study. Arch Neurol 1993 Jun;50(6):575-581.
- 9. Kalita J, Chandra S, Kumar B, Bansal V, Misra UK. Cerebral Venous Sinus Thrombosis From a Tertiary Care Teaching Hospital in India. Neurologist 2016 May;21(3):35-38.
- 10. Ramdasi R, Mahore A, Kawale J, Thorve S. Cerebral venous thrombosis associated with tuberculous meningitis: a rare complication of a common disease. Acta Neurochir (Wien) 2015 Oct;157(10):1679-1680.
- 11.Kleinschmidt-DeMasters B, Mazowiecki M, Bonds LA, Cohn DL, Wilson ML. Coccidioidomycosis meningitis with massive dural and cerebral venous thrombosis and tissue arthroconidia. Arch Pathol Lab Med 2000 Feb;124(2):310-314.
- 12. Nieuwland R, Berckmans RJ, McGregor S, Böing AN, Romijn FP, Westendorp RG, et al. Cellular origin and procoagulant properties of microparticles in meningococcal sepsis. Blood 2000 Feb;95(3):930-935.
- 13. Kornelisse RF, Hazelzet JA, Savelkoul HF, Hop WC, Suur MH, Borsboom AN, et al. The relationship between plasminogen activator inhibitor-1 and proinflammatory and counterinflammatory mediators in children with meningococcal septic shock. J Infect Dis 1996 May;173(5):1148-1156.
- 14. Stam J. Thrombosis of the cerebral veins and sinuses. N Engl J Med 2005 Apr;352(17):1791-1798.
- 15. Algahtani HA, Abdu AP, Shami AM, Hassan AE, Madkour MA, Al-Ghamdi SM, et al. Cerebral venous sinus thrombosis in Saudi Arabia. Neurosciences (Riyadh) 2011 Oct;16(4):329-334.
- 16. de Bruijn SFTM, de Haan RJ, Stam J. Clinical features and prognostic factors of cerebral venous sinus thrombosis in a prospective series of 59 patients. Journal of Neurology, Neurosurgery and Psychiatry 2001 01;70(1):105-108.
- 17. Nürnberger W, v Kries R, Böhm O, Göbel U. Systemic meningococcal infection: which children may benefit from adjuvant haemostatic therapy? Results from an observational study. Eur J Pediatr 1999 Dec;158(S3)(Suppl 3):S192-S196.
- 18. Manios SG, Kanakoudi F, Maniati E. Fulminant meningococcemia. Heparin therapy and survival rate. Scand J Infect Dis 1971;3(2):127-133.
- 19. Feinstein DI. Diagnosis and management of disseminated intravascular coagulation: the role of heparin therapy. Blood 1982 Aug;60(2):284-287.
- 20. Preter M, Tzourio C, Ameri A, Bousser MG. Long-term prognosis in cerebral venous thrombosis. Follow-up of 77 patients. Stroke 1996 Feb;27(2):243-246.