Etiology and management of bilateral vocal cord paralysis (VCP) in pediatrics differs from that in adults where it constitutes a part of the larger clinical entity called ‘bilateral vocal cord immobility’.1,2 Congenital laryngeal paralysis or bilateral abductor VCP presenting as neonatal stridor is rare and often requires prolonged mechanical ventilation.3,4 Syndromic as well as non-syndromic varieties of bilateral congenital laryngeal paralysis are documented in the Western literature.5,6 To the best of our knowledge, there are no reported cases from the Middle East. Here we report an Omani family wherein otherwise healthy and asymptomatic parents had three male newborn babies and one maternal uncle having features of congenital bilateral VCP suggesting X-linked recessive inheritance.
Case Report
A full-term male newborn (weight 3.2 kg) was born by an elective cesarean section (indication: previous cesarean section) to a gravida-8, para-7 mother aged 32 years. The baby had severe inspiratory stridor and central cyanosis soon after birth. Endotracheal intubation was done given the worsening respiratory distress in the labor room and mechanical ventilation was subsequently initiated. Upon ear, nose, and throat evaluation with direct laryngoscopy, the vocal cords were found in the paramedian position with limited movements suggesting bilateral abductor vocal cord palsy. Flexible fiberoptic laryngoscopy
was done to confirm the diagnosis. There was no evidence of laryngomalacia. The baby was ventilated for four days and weaned to nasal continuous positive airway pressure. Feeding was started on day two and full enteral feeds achieved by day six. Blood culture showed growth of Klebsiella pneumoniae and antibiotics were administered according to the sensitivity pattern (cefotaxime 150 mg/kg/day and amikacin 15 mg/kg/day). Additional investigations to evaluate congenital stridor including chest
X-ray, cranial ultrasonography, echocardiography, skeletal survey, and routine laboratory work-up were normal. The baby had no dysmorphic features on physical examination; there was no evidence of any other organ dysfunction. Antenatal history for the present pregnancy was uneventful, and the mother had no existing or previously treated medical or surgical conditions.
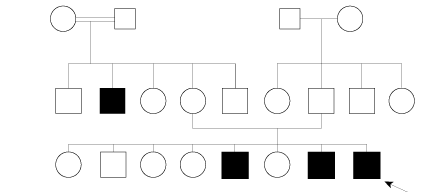
Figure 1: Pedigree chart showing the index case and affected of other male members of the family suggesting an X-linked recessive inheritance pattern.
Other causes of VCP like traumatic or forceps delivery, mediastinal surgery, and ligation of patent ductus arteriosus, brainstem anomalies, and intracranial bleeding were not present in our case, and hence the diagnosis of idiopathic congenital bilateral vocal cord paresis was made. Significantly, there was a history of neonatal stridor in two previous siblings, both male. Both required respiratory support in the form of endotracheal intubation and mechanical ventilation for one and two weeks, respectively. The first child was diagnosed with bilateral VCP in India and no underlying defects were found on magnetic resonance imaging of the brain. Both had undergone fiberoptic laryngoscopy and respiratory symptoms resolved completely towards the end of first year of life without the need for tracheostomy as stated by parents. The elder siblings (aged six and 2.5 years, respectively) were asymptomatic with a normal voice. The parents had no respiratory symptoms, and there was no history of stridor at birth or dysphonia during infancy or childhood. A pedigree analysis for the family was done [Figure 1]. One of the maternal uncles of the index case had
a history of noisy breathing at birth that resolved over several months according to the mother. We were unable to contact the uncle. The current child is being regularly followed-up. He is now
eight months old and had normal vocal cord movements documented at six-months. His recovery was earlier than his elder siblings.
Discussion
The diagnosis of idiopathic laryngeal paralysis in our case was straightforward as there was no history of a difficult delivery or other obvious causes for the presentation. There was a history of stridor at birth in two elder male siblings who were evaluated using laryngoscopy. The parents did not consent to any further investigations or invasive interventions (i.e., tracheostomy) in any child. However, the baby was successfully weaned from the ventilator as early as four days after birth. There were no congenital malformations or other comorbidities previously reported in the literature [Table 1].
Table 1: Neonatal stridor due to congenital bilateral vocal cord paralysis: clinical associations reported in literature.6,7
- Laryngomalacia
- Subglottic stenosis
- Subdural hematoma
- Central nervous system malformations including Arnold Chiari malformation
- Arthrogryposis
- Facial dysmorphic features
- Velopharyngeal insufficiency
- Hypotonia/myopathy
- Ear deformities/sensorineural hearing loss
|
VCP is the second most common cause (10–15%) of neonatal stridor, after laryngomalacia. However, there are fewer than 50 cases of neonatal presentations of bilateral ‘idiopathic’ congenital VCP identified in the English literature.6 Other causes of neonatal stridor are nerve injury during delivery, cardiothoracic surgery, and underlying neurological conditions.8,9 Radiographic evaluation including neuroimaging and flexible fiberoptic laryngoscopy should be done in all cases. Anomalies that need to be excluded in these cases include head and neck masses causing compression, Arnold Chiari malformation, meningoencephalocele, and hydrocephalus. Laryngeal electromyography wherein the electrodes are placed inside larynx is cumbersome in the pediatric age-group and therefore not tried in our case.2
Infants with congenital VCP are usually symptomatic within the first 48 hours of life and often complicated by aspiration pneumonia. Tracheostomy is required in up to 70% cases with bilateral VCP but was avoided in our case.1,4 The decision was based on parental concerns, as well as the fact that the baby could be weaned from ventilator early, and the siblings had a spontaneous recovery of vocal cord mobility. Several workers in the past have observed that only 35% of the children with bilateral VCP can be managed without tracheostomy.4,8,10 More recently, the trend is towards conservative management, especially in neonatal presentations.6,11 Recovery may take anywhere between six months and 11 years.10 Therefore, a waiting period of 12–18 months is recommended before contemplating definitive surgical intervention in children.
The earliest description of congenital bilateral vocal cord dysfunction by Plott dates back to 1964 and X-linked inheritance was postulated subsequently by other authors.12 There were no associated dysmorphic features in the original description. Association with psychomotor delay was probably due to severe hypoxic events during/after birth. Familial bilateral VCP in the absence of other associated features [Table 1] described in our report is not a well-known entity.5 It has aptly been called ‘Plott syndrome’ after Dwight Plott.7,13 Literature on clinical presentations and underlying genetic defects in congenital VCP are reviewed in a recent paper.14 The genetic basis for non-syndromic familial forms of VCP reported to date include a gene locus on 6q16 and inversion of chromosome 13 reported by Hsu et al.14 An extensive literature review on neonatal cases of congenital VCP is published.6 However, our literature review suggested that no cases of familial bilateral VCP have so far been reported from the Middle East, which makes our report unique. Four males in two successive generations in a family were affected at birth with a complete resolution of symptoms during infancy without complications like aspiration pneumonia, dysphonia, or long-term respiratory symptoms [Figure 1]. Tracheostomy seems to be unnecessary in familial non-syndromic cases (as observed in our case study) when compared to non-familial cases.11
We could not do genetic analysis in any of our patients due to cultural issues leading to reluctance to give consent. However, based on pedigree analysis, X-linked recessive inheritance is most likely, which is similar to the recent report of clustering in a family.5 Adult-onset bilateral abductor paralysis (Gerhardt syndrome) has also been reported in some families but is not X-linked.15 Moreover, the clinical presentation is at birth in Plott syndrome.
Conclusion
The diagnosis of bilateral VCP should be considered in cases with neonatal stridor. A detailed family history should be sought. Endoscopic laryngeal examination remains the diagnostic gold standard. In this report, we have described familial congenital bilateral vocal cord paresis with spontaneous recovery during infancy in an Omani family. The condition appears to be X-linked in inheritance.
Disclosure
The authors declared no conflicts of interest.
Acknowledgements
We thank the team of specialists in the ENT department for confirming our findings using fibreoptic laryngoscopy.
references
- 1. Brake MK, Anderson J. Bilateral vocal fold immobility: a 13 year review of etiologies, management and the utility of the Empey index. J Otolaryngol Head Neck Surg 2015 Jun;44:27.
- 2. Chen EY, Inglis AF Jr. Bilateral vocal cord paralysis in children. Otolaryngol Clin North Am 2008 Oct;41(5):889-901.
- 3. Holinger LD. Etiology of stridor in the neonate, infant and child. Ann Otol Rhinol Laryngol 1980 Sep-Oct;89(5 Pt 1):397-400.
- 4. Jabbour J, Martin T, Beste D, Robey T. Pediatric vocal fold immobility: natural history and the need for long-term follow-up. JAMA Otolaryngol Head Neck Surg 2014 May;140(5):428-433.
- 5. Amos L, Quintero D, Veith R, Trapane P, Beste D, Gershan W. A rare presentation of neonatal stridor. Clin Pediatr (Phila) 2012 Mar;51(3):294-296.
- 6. Nisa L, Holtz F, Sandu K. Paralyzed neonatal larynx in adduction. Case series, systematic review and analysis. Int J Pediatr Otorhinolaryngol 2013 Jan;77(1):13-18.
- 7. Online Mendelian Inheritance in Man. Vocal cord dysfunction, familial plott syndrome [cited 2016 April 16]. Available from: http://www.omim.org/entry/308850.
- 8. Miyamoto RC, Parikh SR, Gellad W, Licameli GR. Bilateral congenital vocal cord paralysis: a 16-year institutional review. Otolaryngol Head Neck Surg 2005 Aug;133(2):241-245.
- 9. Garcia-Lopez I, Peñorrocha-Teres J, Perez-Ortin M, Cerpa M, Rabanal I, Gavilan J. Paediatric vocal fold paralysis. Acta Otorrinolaringol Esp 2013 Jul-Aug;64(4):283-288.
- 10. Daya H, Hosni A, Bejar-Solar I, Evans JN, Bailey CM. Pediatric vocal fold paralysis: a long-term retrospective study. Arch Otolaryngol Head Neck Surg 2000 Jan;126(1):21-25.
- 11. Lesnik M, Thierry B, Blanchard M, Glynn F, Denoyelle F, Couloigner V, et al. Idiopathic bilateral vocal cord paralysis in infants: Case series and literature review. Laryngoscope 2015 Jul;125(7):1724-1728.
- 12. Plott D. Congenital laryngeal-abductor paralysis due to nucleus ambiguus dysgenesis in three brothers. N Engl J Med 1964 Sep;271:593-597.
- 13. McDonald D. Anaesthetic management of a patient with Plott’s syndrome. Paediatr Anaesth 1998;8(2):155-157.
- 14. Hsu AK, Rosow DE, Wallerstein RJ, April MM. Familial congenital bilateral vocal fold paralysis: a novel gene translocation. Int J Pediatr Otorhinolaryngol 2015 Mar;79(3):323-327.
- 15. Amir I, Crow YJ, Morar P. Adult-onset familial vocal fold paralysis. Ear Nose Throat J. 2015; 94(9):E1-E3.