Writing a research proposal can be a challenging task, especially for inexperienced researchers. This article explains how to write a research proposal to apply for funding, specifically, a proposal for The Research Council (TRC) of Oman.
A research proposal is a concise and coherent description of a planned research project.1 It should contain all of the information the evaluators will need, such as the background information about the topic, the project’s significance and objectives, and the methods you will employ to achieve the objectives and budgetary details.
The components of research proposals are similar worldwide, with small differences that depends on the funder’s requirements. In TRC, three research proposal application forms are used for three different research grants awarded to individual researchers:
- Open Research Grant (ORG), awarded to researchers holding a Ph.D. degree (or equivalent).2
- Graduate Research Support Program (GRSP) a small grant intended to support predoctoral researchers.3
- Faculty-mentored Undergraduate Research Award Program (FURAP), a small grant for undergraduate students.4
The differences in the contents and length of these applications are attributed to the nature and requirements needed for each grant. The application forms are completed and submitted electronically on TRC website. The contents of these applications forms are summarized in Table 1. After submission, the proposal passes through several stages [Figure 1].
Table 1: Content of different research proposal applications forms at The Research Council.
|
Open Research Grant (ORG) |
Title
Executive summary
Introduction and statement of the problem or project
Literature review and analysis of related work
(with references)
Description of the benefits to Oman
Outline of proposed activities and research methodology
Academic, scientific, and/or innovative significance
Expected economic impacts
Expected social, cultural, educational, and welfare benefits
Cost analysis
Duration of the project |
Ph.D. holder or equivalent |
No cap |
|
Graduate Research Support Program (GRSP) |
Executive summary
Background
Specific aims
Significance and career goals
Research design and methods
Execution plan
References
Budget |
Master/Bachelor degree holders
Postgraduate students |
5 000 |
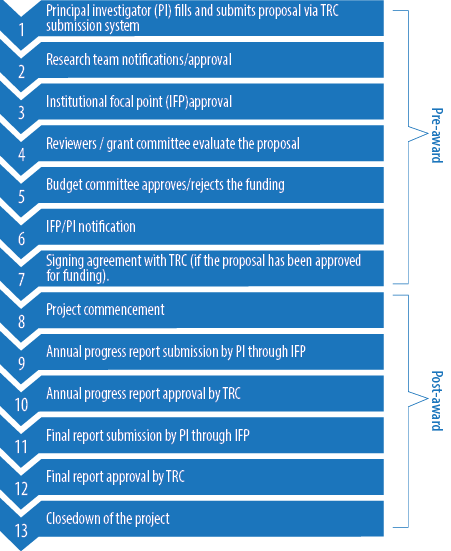
Figure 1: General overview of the pre- and post-award process of a research proposal submitted to The Research Council (TRC).
Before you start
Good research starts with a good idea. To identify a good research idea, you should ask yourself several questions:
- What do you find intriguing in your field?
- What do you want to add to your field?
- Is this project a novel contribution? Does it differ from existing research?
- If your answer to question 3 is “no,” but you believe that your approach to the problem is different from existing methods, why do you believe that your approach will have a better outcome?5
After you answer the questions, you can begin formulating a research question. However, not having all of the answers to those questions indicates that you are not ready and that you will need to perform an in-depth review of the literature on your topic to compose researchable questions. Composing an important question that you can answer is a challenging task, because it is difficult to find a research question that can be transformed into a feasible and valid study plan.6 Formulating a research question is discussed in more detail on a separate section.6,7
Title of the proposal
The title of the research proposal is important to evaluators. It piques the reader’s interest and, therefore, should be selected carefully. The title should not be too long but should be concise, descriptive, and informative. Try to avoid unnecessary words, such as “investigation into…”, “study of…”, and do not overstate expected outcomes in the title.7–9 Some examples of good and bad titles are listed in Box 1.
Box 1: Examples of good and bad research titles.
|
“Anxiety, depression and diabetes”
(too brief, not informative).
“Study of the role of anxiety and depression in onset of diabetes in interior regions of Sultanate of Oman: A Prospective study”
(not concise, contains unnecessary words).
“A prospective study of the role of anxiety and depression in onset of diabetes type II”
(informative, concise and not too long). |
Formulating research questions
Research questions differ from hypotheses and objectives. The research question is the question that the project sets out to answer; it guides and centers your proposed research, whereas a hypothesis postulates a relationship between two or more variables. In other words, it translates the research question into a theory of expected outcomes. Objectives are detailed descriptions of the project’s purpose and expected achievements.10,11
To develop a strong research question, you should ask yourself the questions listed above. Choose a topic of interest and conduct preliminary research. Focus on recent publications to find out what research has already been performed on this topic.
Good research questions have several features, including the following:
- Novel and original: Do not copy a research question that already has an answer.
- Significant merit: Research questions must have scientific value and should add significant information to the literature on that particular topic.
- Relevant to the context: in which the research is conducted. Strong research questions should offer a solution to a problem in the community.
- Ethical: Before you start writing a research proposal, ask yourself: What are the potential harms and benefits to the participants? Harm could be physical (such as that caused by medical interventions) or mental (such as breaches of confidentiality). All of these issues will be assessed by an ethics committee (see “Ethical Considerations” section).
- Clear and reasonably short: Posing questions that are unclear or too long gives the reader the impression that you lack knowledge of the topic. If you have too long a question, you can break it down into multiple questions to make it simple and understandable.
- Feasible: The feasibility of a research question is crucial when applying for a grant from a funder. The question must be within your ability to handle in terms of resources needed and project duration. It is good practice to put a contingency plan in place to anticipate possible problems.6,10
Some examples of research questions are illustrated in Box 2.
Box 2: Examples of research questions.
|
“Does smoking contribute to heart diseases in diabetic patients?”
(This research question has a weak scientific value because smoking is well known to be a risk factor for heart diseases in normal individuals, so it does not add anything novel to the topic).
“Do the diabetic patients with major depression are more likely to have higher cardiovascular risk factors?”
(This question is relevant, it has a value to the health care, feasible, and clear). |
Hypothesis
The hypothesis is a fundamental component of quantitative research; it predicts a relationship between two or more variables. Like the research question, a strong hypothesis should have several important features. It should be novel and original, measurable by the proposed methodology, fit the research interests of the principal investigators, have reasonable predictions of the relationship between the variables, and relevant to the context of the research [Box 3].6,11 You can include more than one hypothesis in your research proposal, but pay attention to the adequacy of the sample size.8
Box 3: Box 3: Research hypothesis examples.
|
“Elevated level of inflammatory mediators is associated with major depression in diabetic patients”(This is a good hypothesis, based on several evidences. Elevated circulating inflammatory markers: (a) predict the development of type 2 diabetes, and (b) may have a role in the biology of major depression).Any hypothesis not based on scientific evidences is considered a weak hypothesis.
|
The development of a good hypothesis will offer insight into the specific objectives of a study. Remember that if the research proposal is based on a weak hypothesis or unfeasible, it may be rejected.
Executive summary
The executive summary is a brief overview of the research proposal. It should provide the reader with the main points and the conclusion of your proposal. The required length varies; it may be between 200 and 400 words or be as long as one A4 page, depending on funder requirements. It is best to keep the summary short and clear. It should include an introduction that provides the reader with background information (such as the problem and its significance, the research question, and the rationale for the study), objectives, the target population and study sample, a brief description of your methodology (your approach to finding a solution), and the study’s expected impact. It should not contain references, abbreviations, citations, tables or figures.7–9, 12
Introduction and statement of the problem/project
The principal purpose of the introduction and statement of the problem are to explain the meaning of your research, present the problem, and provide a strong argument for why the study is necessary. It should be presented in a focused manner rather than in a general context. Try to “enlarge” your research question and pose it in a realistic way as a “hot” topic. Frame the problem by giving recent numbers or percentages of individuals affected globally and locally, and well as the incidence (rate of occurrence) and prevalence. Clearly describe the effects of a health problem, for example, on individuals or society (e.g., poor quality of life, health care or society costs). Finally, it is advisable to select a problem that national or international organizations consider a research priority.
The introduction and statement of the problem includes the following elements:
- A brief introduction to the topic.
- A discussion of the problem’s significance.
- Novelty of the research (at least in a local context).
- Reasons for conducting the study.
- Expected outcomes and benefits of the study.
Literature review
Authors should realize that the literature review differs from the introduction to the problem and that they should be separated from each other. Essentially, a literature review is an extensive survey of the research that has already been conducted on the chosen topic. It is an essential component of a research proposal. It allows you to discover what has been written about the topic, state what each source contributes to the topic, examine the relationship between various contributions, outline contradictions, and identify any lacunas or unresolved questions.
Researchers should invest significant effort in the literature review because it exhibits your knowledge of the topic and allows you to develop new insights into your research. Stated simply, you should convince
the proposal’s evaluators that your project will make a significant original contribution to the subject area.
The literature review should be well organized. To accomplish this, imagine that you are writing a story. The events of the story should take place in the correct order; therefore, use subheadings to bring a clear sequence and coherence to your narrative. Start with the main theme and break it down into smaller parts, avoid the repetition of words or sentences, cite any influential papers, read current papers to keep up with recent developments on the research topic, critically evaluate those papers and formulate a framework showing the relationship between them. Ensure that these papers are of sufficient value to merit citing; avoid irrelevant, trivial references or secondary sources. Finally, include in your review all of the factors stated in the section on specific objectives.6–11
Medical references can be obtained from many search engines, which offer a comprehensive resource to researchers and healthcare providers, such as the National Library of Medicine, OmniMedicalSearch, PubMed, and many others.
Aims and objectives
The aim section is a broad statement describing the goals of the project or the overall purpose of the research project, followed by specific objectives.
Specific objectives are statements that define the measurable outcomes and detail the main aim or purpose of the project.9,10
Both the aims and objectives should be related to the study’s hypothesis. It should be measurable, achievable, realistic, and time-constrained. It is crucial that the aims and objectives have these features because objectives provide reviewers with a clear picture of what you want to accomplish, form the foundation for the rest of the proposal, and are used to assess the adequacy and appropriateness of the proposed methods. Moreover, a strong aim and objective statements should contain active verbs, such as “examine”, “construct”, “classify”, and “develop”.7,9–11 If you have more than one objective, it is advisable to order them numerically. An example of an aim and specific objectives is given in Box 4.
Box 4: Example of a strong aim and specific objectives.
|
The main purpose of this study is to determine the association of major depression with cardiac risk factors in type II diabetic patients.The specific objectives of this study are to:
1. Assess the cardiovascular risk factors in patients with type II diabetes.
2. Mesaure the depression levels in patients with type II diabetes.
3. Associate the depression level with cardiac risk factors.
4. Measure inflammatory mediators (e.g., interleukin-1) in type II diabetic patients.
5. Correlate the level of these inflammatory mediators with depression levels.
|
Research methods
The research methods section is a description of how the research objectives can be achieved and the project completed. When you start explaining your methods, always bear in mind the project’s specific objectives and write in the same order as the description of objectives.6,9
You should provide sufficient evidence to convince the evaluators that your approach is the most appropriate way to engage the problem. Supplement the description of your methodology with references to trusted and useful sources, and do not give the evaluators reason to criticize your approach. To avoid criticism when selecting a method, ask yourself the following question: Is this method appropriate, accurate and sensitive, reproducible, feasible, reliabl, and up-to-date?
In this section, you can include the study design and what type of data you will collect and how you will analyze these data. In sum, you should explain what type of statistical procedures will be used. For more information, please refer to the Equator Network website.13
Sample size calculation is a crucial part of the methods section and justifications of the estimated sample size should be clearly presented. There are many free automated programs and websites that can calculate sample size. The sample size required for a study depends on the aim and type of the study.6,12
Finally, inclusion and exclusion criteria should be reasonably discussed if your study includes human participants, ask yourself two questions: What are the criteria for inclusion or selection? What are the criteria for exclusion?9 An explanation of how you plan to eliminate any bias in the selection of participants should be clearly stated.6,7,12
Brief overview of study design
Study design should be incorporated into the methodology sections of most research proposals, including those for TRC. The selection of a study design depends on the research question, and your design should be in line with the purpose of the study and hypothesis. In this section, we will give a brief account of the different types of study design. Study design types can be divided into two major categories: observational and experimental studies. In observational studies, subjects are only observed and no intervention is introduced, whereas experimental studies involve an intervention.6
Observational studies can be divided into four main types: case series, cross-sectional, case-control, and cohort studies.
Case series studies: These involve a small number of patients who have experienced unusual events or cases that have developed unusual characteristics. A study relaying observations of the adverse effects of a vaccination would be of the case series type. This kind of study does not include control subjects and is not based on a hypothesis, but can generate a hypothesis for further studies. Good examples of these studies include a study on the relative incidence of febrile convulsions two weeks after measles vaccination,14mumps vaccination, and aseptic meningitis.15
Cross-sectional studies: In these studies, subjects are recruited, and data are collected at one point in time rather than over a longer period. They analyze existing conditions. They are useful for healthcare planning and are used to measure the prevalence of both risk factors and their potential outcomes. The major limitation of cross-sectional studies is their lack of temporality; it is not clear whether the risk factor preceded the outcome or vice versa.6,12 An example of a cross-sectional study would be a study investigating the prevalence of breast cancer in a population.
Case-control studies: These assess a health problem in relation to several risk factors. They involve two groups of individuals: the first group, suffering from a particular disease or condition (patients or cases), and another group, unaffected by the disease or condition (the control group). There are different types of case-control studies. The choice of type depends on how the cases and control data are to be gathered. For example, in incidence-density case-
control studies, new (incident) cases will be enrolled and the control group will be selected at the time when the incidence case occurs in the study base. In a case-crossover design, the cases, measured at a different time, serve as their controls. Although case-control studies are known for their efficiency in studying rare outcomes, they may suffer from information bias due to the retrospective data collection regarding risk factors.6
Cohort studies: These assess numerous health effects of a single risk factor. Therefore, cohort study subjects consist of a group of people selected based on possessing the risk factor and then followed prospectively over a given period. This type of study is sometimes called a “prospective study”. Nevertheless, it is worth noting that some cohort studies are referred to as “historical cohort studies” or “retrospective cohort studies” because historical information is collected and analyzed. Nonetheless, the direction of the inquiry is still prospective, following a possible cause or risk factor to an outcome over time. For example, a study to follow-up the effect of exposure to certain toxic substances and development of cancer over time.
Cohort studies, in contrast to case-control studies, are suitable for studying rare risk factors and inefficient for studying rare outcomes. Prospective cohort studies are usually more expensive and time-consuming than case-control studies.6,10
All observational studies face a major limitation, which is the presence of confounders (a third variable related to both the outcome and the risk factor). Known confounders can usually be controlled for in the study design using restriction or matching. Alternately, they can be controlled for in the analysis by computing a multivariate regression analysis. However, residual confounding due to unmeasured or unknown confounders remains a potential bias that requires careful interpretation of the results.6,10
Experimental studies are less susceptible to confounding because the exposures (interventions) are assigned to the participants, but they are surrounded by greater ethical constraints. True experimental design is widely used in healthcare research. For example, randomized clinical trials are considered an experimental research design. If random assignment was carried out accurately and sufficiently, randomization will make the groups comparable in terms of both known and unknown confounders. Quasi-experimental methods may be used when it is not possible to randomize individuals or groups into treatment and control groups. There are several other types of experimental research design.16,17 However, discussing of these goes beyond the scope of this review.
Social and economic impact
Social and economic impact is one of research proposal evaluation criteria assessed by the grant committee at TRC. The social and economic impact of a research project depends on the scale and scope of the study. Small grant research (such as GRSP, FURAP) usually has low impact. However, when requesting medium-big grants (via ORG for example), this criterion must be thoroughly justified.
When you are writing a research proposal, despite the limited scope of your research, do not indicate that your research has no social or economic impact, but explain, in a realistic way, that your work has some social and economic impact and who could potentially benefit from your research, either in the short- or long-term. Remember that an excellent research proposal should demonstrate a contribution to society and the economy.
TRC is a governmental institution; in the coming years, the government will place an increasing emphasis on the need for evidence of economic and social returns from its investment in research. Therefore, all TRC-funded research should make the biggest possible impact on the economy. For example, in the last several years, rising health care costs of non-communicable diseases have become an issue and a burden to the economy of the country, health research should focus on reducing the cost of health care of these diseases.18,19
Research budget
Budget is a key factor of most research grant proposals, categorizing all of the expenses necessary to the success of your project. A detailed budget should include all of the costs of any personnel, equipments, supplies, and activities required for the project.
TRC has developed an electronic spreadsheet from which you can choose the required items from different categories, including justifications for requiring certain items.
Budget information provides clues to reviewers about whether the proposed project is feasible. Additionally, it is important to the funder, as an overstated budget may be a factor in rejecting the proposal. Thus, overestimating or underestimating the budget is a problem in research proposal preparation. Therefore, before you start preparing a research budget, you should carefully read the funding rules and guidelines, then follow funder instructions as closely as possible. Make it clear that all budget requests are reasonable and consistent with funder rules and guidelines. Provide reasonable justifications for each selected item, and explain why each of these items is needed to complete the proposed project.
Finally, it is advisable to finalize the budget section after completing the research plan. This will allow you to outline a more accurate and realistic budget.
Table 2: Some common mistakes observed in health research proposals submitted to The Research Council (TRC).
- No significant merit, lack of originality or novelty.
|
- Write the significance of your research by looking into the general or specific contributions to society, individuals, or current scientific knowledge.
|
- No hypothesis or a weak hypothesis, lack of research questions.
|
- Ensure that the research question(s) and/or hypothesis are original (see formulating research questions).
|
- Too many/unrealistic/overly ambitious objectives, which cannot be achieved within the given timeframe.
|
- Develop a research plan and timeline for each objective. Ensure that the allocated time is sufficient to accomplish the objectives, take in account unexpected influences that may delay the research progress.
|
- The purpose of the research is not adequately justified.
|
- The statement of the problem should be clear and explain the needs of your study.
|
- Failure to stay focused on the research question, the proposal rambles on without a clear sense of direction.
|
- Before writing a research proposal, you should be familiar with writing skills. Reading articles in this field and attending workshops are very helpful.
|
- Inadequate knowledge of the literature.
|
- The research activities should take place in the correct order; use subheadings to bring a clear sequence and coherence to your narrative.
- Refer to the section "literature review" for more detail.
|
- Technical inadequacies in methodology, such as insufficient methodological detail, unclear data analysis, poor research design, or unreliable methods.
|
- You should provide sufficient evidence to convince the evaluators that your approach is the most appropriate way to engage the problem. Use trustworthy sources.
|
- A poorly written proposal containing too much detail on minor issues, but not enough detail on major issues. The proposal may also contain incorrect or trivial references.
|
- Refer to the section "literature review" and "Proofread your research proposal" for more detail.
|
- An inflated budget without sufficient justification.
|
- A strong justification is required for each category of the budget. Read the funders guidelines, do not exceed the limits set.
|
- The TRC’s guidelines are not followed.
|
- Read the funders guidelines before writing a research proposal.
|
- The host organization is not well prepared to accommodate the project.
|
- Develop research proposal that can be accommodated by your (or your collaborators) institutional research facilities.
|
Collaborations
The section discussing collaborations is essential to a research proposal. Collaborators can be local or international, but working with reputable and well-funded researchers will make it more likely that the evaluators will have confidence in your ability to complete the project successfully. Provide the funder with a letter of support from your collaborators. The stronger the letter, the better your chances of receiving funding.
Ethical considerations
There are three broad ethical principles that should be observed prior to conducting any research involving human participants:
- Beneficence and non maleficence: simply, maximize benefit and minimize harm. Harm can be physical (such as from research interventions) or mental (such as breaches of confidentiality, stigmatization, and discrimination and to lesser extent culturally or religiously sensitive survey questions).
- Equality: a fair balance of risks and benefits.
- Dignity and autonomy: Participate decision must be treated with respect and allowed to exercise autonomy.20,21
All TRC-approved research proposals involving human or animal are subjected to ethics committee approval before releasing the requested fund. The ethics committee ensure that the research is ethically acceptable and that the welfare and rights of research participants are protected. Therefore, when writing a research proposal, you should ensure that:
- No risk to the participants or risks to participants are minimized.
- Written informed consent is taken from participants or their legally authorized representatives.
- Confidentiality is adequately maintained.
Respect for people also covers the interests of researchers. These include authorship and intellectual property interests, and collegial and professional interests.22
The primary author in TRC research proposal application form is the principal investigator. The principal investigator owns the authorship of the work. All principal investigators (authors) should meet the following criteria for authorship, as recommended by the International Committee of Medical Journal Editors (ICMJE): 22
- Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work.
- Drafting the work or revising it critically for important intellectual content.
- Final approval of the version to be published.
- Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
A research proposal is similar to a manuscript, both should be free from any form of plagiarism, duplicate publication, ghost authorship, copyright laws infringement, any form of bias or conflict of interest, fabrication or falsification (manipulation of data to fit the required paradigm),23,24 failure to get ethical approval from approved ethics committee, and failure to obtain informed consent from participants.24 All these are considered research misconduct and may be a reason for disapproval/discontinuation of a research proposal.
Proofread your research proposal
After you complete your research proposal, revise it more than once to make it clear and concise, ask others to critique and edit your work to highlight issues that you have not covered and eliminate mistakes that you did not see. Common mistakes seen in research proposals are given in Table 2. Make sure that your proposal is jargon-free. Remember that jargons in a research proposal may be a fatal mistake.
Conclusion
This article highlights the important aspects of research proposal’s preparations and submission to TRC. Before starting to write a research proposal to TRC, you must read the guidelines carefully and focus on the evaluation criteria assessed by the reviewers and the grant committee. All the elements of research proposal should be written cohesively and arranged according to the guidelines. A successful research proposal should have informative title, self-sufficient and convincing abstract, a novel and feasible research question, up-to-date scholarly and relevant background and rationale, appropriate population and sample size, appropriate measurement and intervention methods, reasonable budget, realistic execution plan, and should address ethical issues.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
references
- 1. Guidelines for Writing a Research Proposal, University of London [cited 2016 Jan 5]. Available from: http://www.law.qmul.ac.uk/docs/postgrad/50379.pdf.
- 2. Open Research Grant guidelines, The Research Council [cited 2015 April 30]. Available from: https://home.trc.gov.om/Portals/0/Guidelines_for_Open_Research_Grant.pdf.
- 3. Graduate Research support guidelines, The Research Council [cited 2016 Feb 10]. Available from: https://www.trc.gov.om/TRCWebsite/files/guidelines-grsp.pdf.
- 4. Faculty Mentored Undergraduate Research Award Program, The Research Council [cited 2016 Sep 7]. Available from: https://home.trc.gov.om/tabid/754/language/en-US/Default.aspx.
- 5. Porter R. Six critical questions to launch a successful grant proposal, NCURA Magazine, 2015. p 49-51.
- 6. Hulley SB, Cummings SR, Browner WS, Grady DG, Newman TB. Designing clinical research. 3rd ed. Philadelphia (PA): Lippincott Williams and Wilkins; 2007, p.1-353.
- 7. Zlowodzki M, Jönsson A, Kregor PJ, Bhandari M. How to write a grant proposal. Indian J Orthop 2007 Jan;41(1):23-26.
- 8. Setiati S. Tips for making a good research proposal. Acta Med Indones 2004 Jan-Mar;36(1):43-47.
- 9. Al-Riyami A. How to prepare a Research Proposal. Oman Med J 2008 Apr;23(2):66-69.
- 10. Haynes RB, Sackett DL, Guyatt GH, Tugwell P. Clinical epidemiology ‘how to do clinical practice research. 3rd ed. Philadelphia (PA): Lippincott Williams and Wilkins; 2006, p. 1-487.
- 11. Farrugia P, Petrisor BA, Farrokhyar F, Bhandari M. Practical tips for surgical research: Research questions, hypotheses and objectives. Can J Surg 2010 Aug;53(4):278-281.
- 12. Grant writing tutorial survey [cited 2016 Jan 10]. Available from: http://www.theresearchassistant.com/tutorial/survey.asp.
- 13. STARD 2015: An Updated List of Essential Items for Reporting Diagnostic Accuracy Studies [cited 2016 Aug 3]. Available from: http://www.equator-network.org/reporting-guidelines/stard/.
- 14. Farrington P, Pugh S, Colville A, Flower A, Nash J, Morgan-Capner P, et al. A new method for active surveillance of adverse events from diphtheria/tetanus/pertussis and measles/mumps/rubella vaccines. Lancet 1995 Mar;345(8949):567-569.
- 15. Miller E, Andrews N, Stowe J, Grant A, Waight P, Taylor B. Risks of convulsion and aseptic meningitis following measles-mumps-rubella vaccination in the United Kingdom. Am J Epidemiol 2007 Mar;165(6):704-709.
- 16. Kirk RE. Experimental design: Procedures for the behavioral sciences. Pacific Grove, CA: Brooks/Cole, 1995.
- 17. Grimshaw J, Campbell M, Eccles M, Steen N. Experimental and quasi-experimental designs for evaluating guideline implementation strategies. Fam Pract 2000 Feb;17(Suppl 1):S11-S16.
- 18. Al-Riyami A. Health Vision 2050 Oman: A Committed Step towards Reforms. Oman Med J 2012 May;27(3):190-191.
- 19. Health vision 2050, Ministry of Health [cited 2016 Oct 16]. Available from: https://www.moh.gov.om/documents/16506/119833/Health+Vision+2050/7b6f40f3-8f93-4397-9fde-34e04026b829.
- 20. The Belmont Report [cited 2015 Dec 4]. Available from: www.edu/irb/pdfs/BelmontReport.pdf.
- 21. Declaration of Helsinki 2013 [cited 2015 Dec 4]. Available from: http://www.wma.net/en/30publications/10policies/b3/17c.pdf.
- 22. Defining the Role of Authors and Contributors, International Committee of Medical Journal Editors [cited 2016 Sep 7]. Available from: http://www.icmje.org/recommendations/browse/roles-and-responsibilities/defining-the-role-of-authors-and-contributors.html.
- 23. Al Lamki L. Ethics in Scientific Publication: Plagiarism and other Scientific Misconduct. Oman Med J 2013 Nov;28(6):379-381.
- 24. Al-Adawi S, Ali BH, Al-Zakwani I. Research Misconduct: The Peril of Publish or Perish. Oman Med J 2016 Jan;31(1):5-11.