|
INTRODUCTION
A large number of chemopreventive agents have been identified in epidemiological and experimental studies, preclinical systems and clinical observations.1,2 However, the toxic side effects produced by some of these agents have limited their extensive use.3 There is therefore a need to identify synthetic or natural compounds that have significant chemopreventive potential without undesirable toxic effects.
Chemoprevention offers a novel approach to control the incidence of oral cancer, an important contributor of cancer morbidity and mortality in the Indian subcontinent.4 Plant products and their active principles have attracted the focus of recent attention as putative chemopreventive agents in the light of their easy availability, non-toxic and anticarcinogenic properties.
A wide number of traditionally important medicinal plants are still used by Indian traditional practitioners for the treatment of cancer. Terminalia arjuna (Roxb.) a deciduous tree of Combretaceae family, has been widely used in Indian system of medicine for various diseases. Terminalia arjuna (T. arjuna) is distributed throughout India and its bark is used as a cardio protective agent in hypertension and ischaemic heart diseases.5 It has been reported that T. arjuna bark showed potent chemopreventive efficacy against N-nitrosodiethylamine-induced liver cancer.
Cancer of the oral cavity is one of the most common malignant diseases worldwide, and the highest incidence (40-50%) of oral cancer was observed in India.6 Epidemiological studies have shown that chewing of betel quid with tobacco is the major aetiological factor of oral carcinogenesis in India.7 7,12-dimethylbenz(a) anthracene (DMBA), a potent carcinogen, can initiate and promote the development of oral carcinoma of the Syrian hamster buccal mucosa and DMBA-induced experimental oral cancer is the most widely-accepted experimental model, since it has many morphological and histological similarities with human oral carcinoma.8 It has been extensively used to test a wide variety of synthetic and natural agents for their chemopreventive potential.
Most phytochemicals are known to exert their anticarcinogenic effects by scavenging oxygen free radicals and enhancing antioxidant levels.9 Assay of circulatory biomarkers has emerged as a reliable method for screening putative chemopreventive agents. Circulatory levels of lipid peroxidation and antioxidants are reliable indicators, because they reflect the bioavailability as well as increased utilization to counter lipid peroxidation.10
This present study aims to evaluate the modifying effects of aqueous extract of T. arjuna bark (TaBet) on circulatory lipid peroxidation, reduced glutathione (GSH), catalase (CAT), superoxide dismutase (SOD) and glutathione peroxidase (GPx) as well as vitamin E and ascorbic acid as biomarkers of chemoprevention, during 7,12 dimethylbenz[a]anthracene (DMBA)-induced hamster buccal pouch carcinogenesis.
METHODS
Terminalia arjuna barks were collected in and around Thanjavur, Tamil Nadu, India during the summer (June-August). The plant was identified and authenticated at the Herbarium of Botany Directorate in Annamalai University, India. A voucher specimen was also deposited in the Department of Botany, Annamalai University. The aqueous extract of T. arjuna bark (TaBet) was prepared according to the method of Hossain et al.11 100 g of dried fine powder of T. arjuna bark was suspended into 250 ml of water for 2 hrs and then heated at 60-65ºC for 30 minutes. The extract was collected and the processes were repeated three times with the residual powder, each time collecting the extract. The collected extract was pooled and passed through fine cotton cloth. The filtrates were evaporated at 40-50ºC in a rotavapour under reduced pressure. An 18% light yellowish semisolid material of T. arjuna bark obtained was stored at 0-4°C until used. A known amount of the residual extracts was suspended in distilled water and was orally administered to the animals by gastric intubation using force-feeding needle during the experimental period.
DMBA was purchased from Sigma-Aldrich Chemical Pvt. Ltd (Bangalore, India). All other reagents used were of analytical grade.
Male golden Syrian hamsters (Mesocricatus auratus) 8-10 weeks old, weighing 80-120 g were purchased from the National Institute of Nutrition, Hyderabad, India and were maintained in Central Animal House, Raja Muthaiah Medical College and Hospital, Annamalai University, India. The hamsters were housed in polypropylene cages at room temperatures (22±2ºC) and relative humidity 55±5% with a 12 hr light/dark cycle in an experimental room. The animals were provided with standard pellet diet (Amrut Laboratory Animal Feed, Mysore Feeds Limited, Banglore, India) and water ad libitum.
The local institutional animal ethics committee (Register number 160/1999/CPCSEA), Annamalai University, Annamalainagar, India, approved the experimental design (Proposal No. 291, dated 29.08.2005). The animals were maintained as per the principles and guidelines of the ethical committee for animal care of Annamalai University in accordance with India National Law on animal care and use.
The animals were randomized into experimental and control groups and divided into four groups of ten animals each. The experimental protocol for the present study is given in Fig. 1. Group 1 (untreated control) animals received neither DMBA nor TaBet. Animals in group 2 were painted with a 0.5% solution of DMBA in liquid paraffin on the left buccal pouches using a number four brush three times per week for 14 weeks. Each application leaves approximately 0.4 mg DMBA.12 Group 3 animals were painted with DMBA as in group 2. In addition, the animals were administered 500 mg/kg body weight TaBet orally three times per week on days alternate to DMBA application.13 Animals in group 4 received only TaBet as in group 3. The experiment was terminated at the end of 14 weeks and all animals were killed by cervical dislocation after an overnight fast. Biochemical studies were conducted on blood and buccal mucosa of control and experimental animals in each group. For histopathological examination, buccal mucosal tissues were fixed in 10% formalin and routinely processed and embedded with paraffin, 2-3 µm sections were cut in a rotary microtome and stained with haematoxylin and eosin.
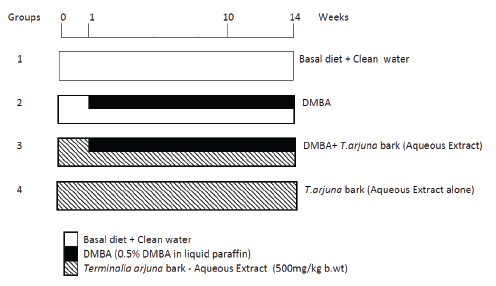
Figure 1: Experimental protocol
The plasma was separated by centrifugation at 3,000 rpm for 15 minutes. After plasma separation, the buffy coat was removed and the packed cells were washed thrice with physiological saline. A known volume of erythrocytes was lysed with hypotonic buffer at pH 7.4. The hemolysate was separated by centrifugation at 10,000 rpm for 15 minutes at 20°C.
The erythrocyte membrane was prepared by the method of Dodge et al.14 modified by Quist.15 Thiobarbituric acid reactive substances (TBARS) were assayed in plasma and erythrocytes according to the methods of Yagi,16 and Donnan,17 respectively. Glutathione (GSH) was determined by the method of Beutler and Kelley.18 Vitamins C and E were measured according to the methods of Omaye et al.19 and Desai,20 respectively. The activities of enzymatic antioxidants, superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx) were estimated by the methods of Kakkar et al.21 Sinha,22 and Rotruck et al.23 respectively.
The data are expressed as mean ± standard deviation (SD). Statistical comparisons were performed by One way analysis of variance (ANOVA), followed by Duncan’s Multiple Range Test (DMRT). The results were considered statistically significant if the p-values were less than 0.05.
RESULTS
Table I shows the incidence of oral neoplasms in the different groups. Exophytic tumors induced by DMBA in the oral cavity of hamsters in group 2 were well differentiated squamous cell carcinomas. The incidence of oral neoplasms in group 2 was 100%, whereas in group 3, only three of the ten animals (30%) developed oral tumors. The mean number of tumors, as well as the tumor burden was significantly lower in group 3 in comparison to group 2. No tumors were observed in the animals of group 1 and 4.
The histopathological features observed in the buccal mucosa of hamsters in the control and experimental animals in each group are depicted in Table 2. The buccal pouches from DMBA-treated hamsters revealed severe keratosis, hyperplasia, dysplasia and well-differentiated squamous cell carcinoma (Group 2). A mild to moderate preneoplastic lesions [hyperplasia (+), keratosis (++) and dysplasia (+)] were noticed in Group 3 animals (DMBA + TaBet).
Table 3 indicates the levels of lipid peroxidation in plasma and erythrocytes of control and experimental animals in each group. Lipid peroxidation was significantly increased in group 2 compared with group 1. In groups 3 and 4, the values were significantly lower compared with group 2.
The levels of antioxidants in plasma, erythrocytes and erythrocyte lysate of experimental and control animals in each group are shown in Table 4. The levels of ascorbic acid, vitamin E, glutathione (GSH), superoxide dismutase (SOD), catalase (CAT) and GPx, were significantly lower in group 2 compared with group 1. In groups 3 and 4, the levels of ascorbic acid, vitamin E, glutathione, as well as the activities of GPx, SOD and CAT were significantly higher compared to group 2.
Table 1: Incidence of oral neoplasm in control and experimental animals in each group (n=10)
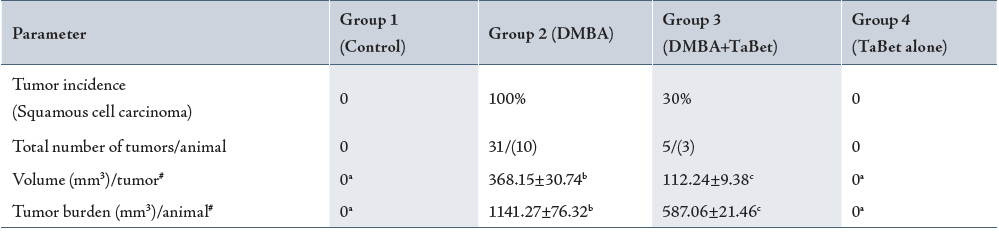
Tumor volume was measured using the formula where D1, D2 and D3 are the three diameters (mm) of the tumor. Tumor burden was calculated by multiplying tumor volume and the number of tumors / animals.
( ) indicates total number of animals bearing tumors.
# Values are expressed as mean ± SD for 10 animals in each group.
Values not sharing a common superscript letter differ significantly at p<0.05 (DMRT).
TaBet – Terminalia arjuna bark aqueous extract
Table 2: Histopathological changes in oral cheek mucosa of control and experimental animals in each group (n=10)
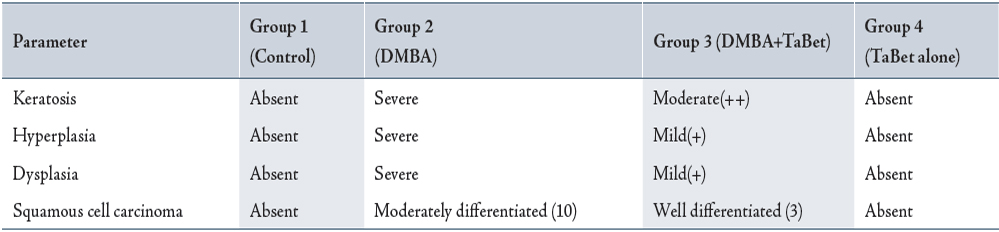
TaBet – Terminalia arjuna bark aqueous extract
Numbers in parentheses indicate total number of animals bearing tumors.
Table 3: Status of TBARS in plasma and erythrocytes of control and experimental animals
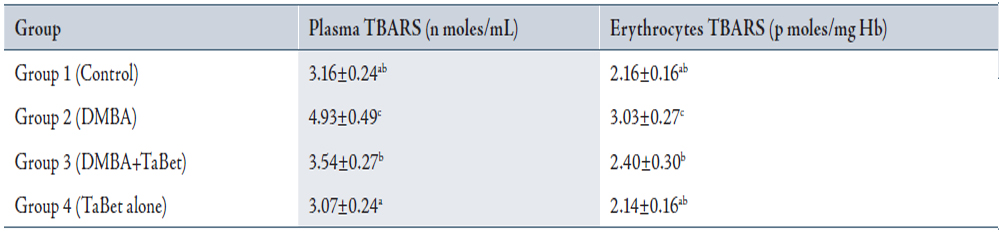
Values are expressed as mean ± SD for 10 animals in each group.
Values not sharing a common superscript letter differ significantly at p<0.05 (DMRT).
TaBet – Terminalia arjuna bark aqueous extract
Table 4: Status of antioxidants in plasma, erythrocytes and erythrocyte lysate of control and experimental animals
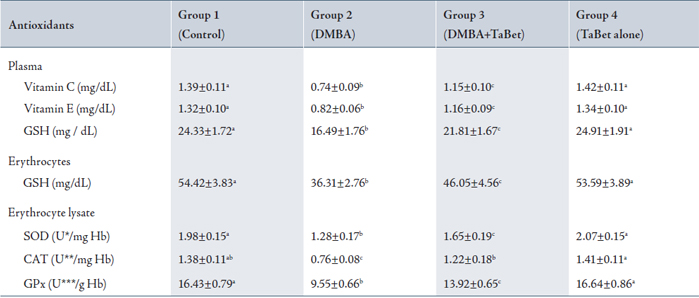
Values are expressed as mean ± SD for 10 animals in each group.
* The amount of enzyme required to inhibit 50% nitroblue tetrazolium (NBT) reduction.
** µ moles of H2O2 utilized / sec;
*** µ moles of glutathione utilized / min.
Values not sharing a common superscript letter differ significantly at p<0.05 (DMRT).
TaBet – Terminalia arjuna bark aqueous extract
DISCUSSION
Oral cancer, a disfiguring disease, has multifactorial eatiologies and occurs predominantly during the sixth to eighth decades of life.24 Oral carcinogenesis is a multifocal disease preceded by distinct premalignant lesion. DMBA-induced precancerous and cancerous lesions in hamsters resemble human oral preancerous and cancerous lesions. Medicinal plants may exert the carcinogenic potential by modulating carcinogen detoxification, inhibiting lipid peroxidation, or by improving in vivo circulatory antioxidants defense mechanism.25 Hyperplasia, dysplasia and severe keratosis, as well as well-differentiated squamous cell carcinoma at 4th week were observe in DMBA-paited animals (group 2). Although mild precancerous lesions were observe in all animals, tumor formation was seen only in the three DMBA-painted animals treated with TaBet (Group 3)
Oral administration of TaBet at a dose of 500 mg/kg body weight to DMBA-painted animals on days alternatae to DMBA paiting for 14 weeks significantly reduced the tumor incidence, tumor volume and tumor burden. Enhance lipid peroxidation assoiciated with antioxidant depletion in circulation is a characteristic finding in malignant transformation. Free radicals, which are highly toxic, traverse membranes and cause deleterious effects at sites far from the tumor.26
The enhanced lipid peroxidation in circulation in hamsters bearing DMBA-induced oral tumors reflects excessive free radical generation exacerbated by a decreased efficiency of host antioxidant defence mechanisms. Increased plasma lipid peroxidation has been reported in several types of cancer patients.27 Erythrocytes are constantly exposed to oxidative stress, and susceptibility of erythrocytes to oxidative stress has been reported in several pathological conditions, including oral cancer.28 Elevated lipid peroxidation in cancer patients may also be correlated to their poor antioxidant system.29 Thus, the observed increase in plasma lipid peroxides in DMBA-painted animals is due to overproduction and diffusion from the damaged erythrocytes and erythrocyte membranes.
Vitamin E, vitamin C and GSH can protect cells and tissues by eliminating or quenching excessively generated Reactive Oxygen Species (ROS) in the body.30 Vitamin C is an essential antioxidant that disappears faster than other antioxidants when plasma is exposed to ROS.31 Vitamin E is the major lipid soluble antioxidant present in plasma and erythrocyte membranes.32 Glutathione, an important cellular reductant, offers protection against free radicals, peroxides and toxic compounds.33 The deficiency of vitamin C, vitamin E and glutathione in the circulation of tumor-bearing hamsters may be due to their increased utilization to scavenge the products of lipid peroxidation. A decrease in the activities of SOD, CAT and GPx, the major cellular detoxifying enzyme systems, has been reported in malignancies.34,35 Our results are in line with these findings.
Oral administration of TaBet not only prevented the tumor formation but also significantly improved the status of lipid peroxidation and antioxidants in DMBA-painted animals, which clearly indicates its potent chemopreventive, antilipidperoxidative, and antioxidant potential in DMBA-induced hamster buccal pouch carcinogenesis. Oral administration of TaBet at a dose of 500 mg/kg body weight significantly prevented tumor incidence, tumor volume, tumor burden and the number of tumors in DMBA-painted hamsters, which indicates that TaBet has a suppressive effect on cell proliferation in DMBA-induced hamster buccal pouch carcinogenesis. The chemopreventive and modulation of circulatory antioxidants effect of TaBet is probably due to the presence of several bioactive chemopreventive principles and their synergistic effects.
CONCLUSION
The results of the present study indicate that T. arjuna may emerge as a putative chemopreventive agent against oral carcinogenesis. Thus, the present investigation warrants further studies to isolate and characterize bioactive chemopreventive principles from the bark of Terminalia arjuna.
ACKNOWLEDGEMENTS
The authors reported no conflict of interest and no funding was received on this work.
|