Dysmenorrhea is defined as difficult menstrual flow1 and refers to painful cramps during menstruation. These cramps can produce uterine pressure more than 60 mmHg, which results in pain.1 Pain is usually in the suprapubic area but may radiate to the back of the legs or lower back and may be accompanied by other symptoms such as nausea, diarrhea, and headache.2 It is a significant health problem in women.3 Primary dysmenorrhea is common among young women and has been reported in 40–50% of them.1,4 A systematic review reported that 75% of adolescents experience dysmenorrhea.5 The prevalence of dysmenorrhea is between 74% to 86.1% in Iran.6 Dysmenorrhea is one of the most common causes of absenteeism from both school and work resulting in large economic, social, and health costs.3,7 Furthermore, dysmenorrhea has disruptive effects on quality of life, usual activities, and individual, family and school adjustment. Patients also need to spend more time performing self-care strategies and looking for medical care to manage the condition.3,7,8
There is some evidence that an increase or imbalance in prostaglandins leads to primary dysmenorrhea.9 Thus, non-steroidal anti-inflammatory drugs (NSAIDs) are the first-line treatment option.10,11 The anti-inflammatory and pain relieving effects of NSAIDs are thought to be due to the inhibition of cyclooxygenase enzyme and reduction in menstrual blood volume.2,11 They may also have a direct analgesic effect on the central nervous system.4 In a recent review of 73 randomized controlled trials, the effectiveness of NSAIDs in the treatment of dysmenorrhea was confirmed.2 Nevertheless, these drugs have some side effects such as gastrointestinal problems and cutaneous adverse drug reactions.12,13 There is some evidence that long-term use of NSAIDs is associated with a degree of cardiovascular risk.2,12,14 Therefore, other treatments such as physical activity and mild exercise have been proposed to
reduce dysmenorrhea.
Two clinical randomized trials showed that stretching exercises were effective in reducing pain intensity in primary dysmenorrhea. They concluded that the effect of regular exercise on dysmenorrhea might be due to the effect of hormonal changes on uterine epithelial tissue or an increase in endorphin levels.6,7 In a 2010 literature review, the authors concluded that exercise reduces the symptoms associated with dysmenorrhea.3
However, data comparing exercise and NSAIDs in the treatment of dysmenorrhea and its outcomes are limited. Additionally, there are some controversies about the effectiveness of exercise on dysmenorrhea, which may be because of different exercise programs. The quality, intensity, and duration of exercise may be associated with different outcomes.3 Therefore, we sought to compare the effect of stretching exercises and mefenamic acid use on the reduction of pain in primary dysmenorrhea and to compare other outcomes such as menstruation characteristics between the groups.
Methods
This randomized clinical trial was conducted on the female students of Mazandaran University of Medical Sciences, Iran, over five months in 2014. The study was approved by the university ethics committee. The sample size calculation was 61 for each group (based on the size effect for exercise of 2.1±2.0 and mefenamic acid of 3.3±1.2, a confidence interval of 95% and power of 80%).2,15 Students living in the university dormitory who had moderate to severe primary dysmenorrhea for more than 50% of menstrual cycles lasting for at least one day and affected their daily activities were included in the study.3,6,7 The diagnosis of primary dysmenorrhea was made based on having characteristics of pain and ruling out any history of pelvic disorders. The characteristics of pain in primary dysmenorrhea are supra-pubic cramps which appeared in first two years of menarche, begin a few hours or just after the onset of menstruation, and last 48–72 hours.16 Students were selected from the dormitory since they had similar nutrition (which may have an effect on dysmenorrhea).17 Patients were excluded if they had irregular menstrual cycles, used an intrauterine device (IUD) or oral contraceptive pill (OCP), undertook regular exercise, had a history of physical or psychological problems, and known secondary dysmenorrhea.3
Eligible students were determined via a convenience sampling using a checklist based on the inclusion and exclusion criteria and were then randomly divided into two interventional groups. All students received an information form and if they agreed to participate, asked to sign a consent form. Demographic and menstrual information such as age, body mass index (BMI), health condition, length and regularity of menstrual cycles, change in bleeding, absence from university, and the need for more analgesics were recorded in a questionnaire. Bleeding change was estimated through the participants’ records about consumed pads at baseline and over the course of the study. Ten professors confirmed the validity of the questionnaire. The test-retest reliability was 0.92. Intensity of pain was measured using a 10 cm visual analog scale (VAS). The VAS is an instrument for measuring characteristics with continuous values that cannot easily be directly measured. The pain VAS is usually a horizontal line, 10 cm in length, defined by descriptive words of “no pain” (score of 0) and “very severe pain” (score of 10). Participants were asked to put a mark on the line that they felt represented the most severe pain that experienced during menstruation. The pain score was determined by measuring the distance between the score of 0 and the participants’ mark in centimeters using a ruler. Severity of pain was classified as mild (< 4 cm), moderate (4–6 cm), and severe (7–10 cm).
The exercise program included a five-minute warm up in a standing position and then six belly and pelvic stretching exercise for 10 minutes. This program was performed for 15 minutes, three times a week in two menstrual cycles (eight weeks). Exercise was not performed during menstruation.6,7 Two trained instructors taught the exercise protocol to the students. Students in the mefenamic acid group received 250 mg capsules every eight hours from the onset of menstruation until pain relief also for two cycles.15 Both interventions were performed during two consecutive cycles. In both groups, the intensity of pain was assessed using the VAS at the end of the first and second menstrual cycles. Follow-up of participants for the assessment of menstruation characteristics was also done in this period. Likewise, participants’ pain intensity was recorded before taking more analgesics whenever needed for additional relief.
Descriptive statistics (mean, standard deviation (SD), frequency, mean difference), Student’s t-test and chi-square test were used for data analysis.
Results
One-hundred and twenty-two participants were equally divided into two groups: the mefenamic acid group (n = 61) and the exercise group (n = 61). Demographic characteristics of participants in the mefenamic acid and exercise groups were compared using the Student’s t-test and chi-squared test and are shown in Table 1.
Table 1: Participants’ demographic characteristics in the mefenamic acid and exercise group.
|
Age, years (mean±) |
21.6±2.0 |
21.3±2.0 |
0.414 |
|
BMI, kg/m2 (mean±) |
21.8±2.9 |
22.6±3.6 |
0.225 |
|
Educational level |
|
MSc and PhD |
16 (72.7) |
6 (27.3) |
0.032 |
|
BSc |
45 (45.0) |
55 (55.0) |
|
|
University year |
|
|
|
|
1st and 2nd |
23 (48.9) |
24 (51.1) |
1.000 |
|
3rd |
38 (50.7) |
37 (49.3) |
|
|
Family history of dysmenorrhea |
|
Yes |
40 (54.1) |
34 (45.9) |
0.095 |
|
No |
10 (33.3) |
20 (66.7) |
|
Data given as n (%) unless otherwise indicated. BMI: body mass index; MSc: Master of Science; PhD: Doctor of Philosophy; BSc: Bachelor of Science.
Table 2: Comparison of pain between the mefenamic acid and exercise group at baseline.
|
Pain intensity, cm |
55.0±20.4
(49.82–60.24) |
60.3±14.5
(56.55–63.96) |
0.104 |
|
Pain duration, days |
1.7±0.9
(1.43–1.90) |
1.8±0.7
(1.60–1.96) |
0.444 |
|
Severity of dysmenorrhea, n (%) |
|
|
0.849 |
|
Moderate |
41 (51.3) |
39 (48.8) |
|
Data given as mean ± SD and (95% confidence interval for mean) unless otherwise stated.
The mean pain intensity in the exercise group was significantly higher than the mefenamic acid group in the first cycle after intervention [Tables 2 and 3]. Pain intensity was significantly different between the groups in the second cycle as the mean difference between baseline and first cycle was higher in the exercise group compared to the mefenamic acid group [Table 4 and Figure 1]. Chi-square analysis showed that the severity of dysmenorrhea and pain duration had no significant difference between the groups during the study [Table 2, 3, and 4].
Table 3: Comparison of pain between the mefenamic acid and exercise group in the first cycle.
|
Pain intensity, cm |
39.0±17.8
(34.46–43.56) |
45.4±19.2
(40.50–50.34) |
0.058 |
|
Pain duration, days |
1.4±0.6
(1.2–1.6) |
1.6±0.8
(1.38–1.79) |
0.131 |
|
Pain reduction between baseline and the second cycle, mean (SE) |
16.0 (2.2) |
14.8 (2.5) |
0.724 |
|
Severity of dysmenorrhea, n (%) |
|
Mild |
22 (52.4) |
20 (47.6) |
|
Moderate |
35 (51.5) |
33 (48.5) |
Data given as mean ± SD and (95% confidence interval for mean) unless otherwise stated. SE: standard error.
Table 4: Comparison of pain between the mefenamic acid and exercise group in the second cycle.
|
Pain intensity, cm |
33.8±17.7
(29.21–38.29) |
31.6±16.03
(27.46–35.68) |
0.477 |
|
Pain duration, days |
1.6±0.7
(1.44 – 1.80) |
1.5±0.7
(1.29–1.69) |
0.378 |
|
Pain reduction between the first and the second cycle, mean (SE) |
5.3 (2.24) |
13.9 (2.1) |
0.007 |
|
Pain reduction between baseline and the second cycle, mean (SE) |
21.3 (2.91) |
28.7 (2.5) |
0.056 |
|
Severity of dysmenorrhea, n (%) |
0.357 |
|
Mild |
33 (45.8) |
39 (54.2) |
|
Data given as mean ± SD and (95% confidence interval for mean) unless otherwise stated. SE: standard error.
Length of menstruation in the first and second cycle was longer in the exercise group, but bleeding changes were not significantly different between groups [Table 5]. Despite the fact that the participants who took additional analgesic drugs in the exercise group were more than the mefenamic acid group, the difference was not statistically significant in the first (61.0% and 39.0%, respectively) or second cycles (64.0% and 36.0%, respectively). Students in the exercise group had more absences (63.0%) than the mefenamic acid group (37.0%) at baseline (p = 0.055), but absences were the same in the two groups after the first and second cycles (50.0% in both groups).
Table 5: Menstruation characteristics in the mefenamic acid and exercise groups.
|
Menstrual intervals, days |
28.4±2.8 |
28.0±4.0 |
28.0±2.7* |
29.2±3.4 |
29.0±4.3 |
29.2±2.9* |
|
Length of menstruation, days |
6.6±1.2 |
5.9±1.3** |
6.2±1.2 |
6.8±1.3 |
6.5±1.1** |
6.1±1.2 |
|
Bleeding, n (%) |
|
|
|
|
|
|
|
Increase |
|
3 (4.9) |
5 (8.2) |
|
6 (9.8) |
9 (14.8) |
|
Decrease |
|
20 (32.8) |
21 (34.4) |
|
16 (26.2) |
21 (34.4) |
Data given as mean ± SD unless otherwise indicated. *p = 0.030, **p = 0.012.
Discussion
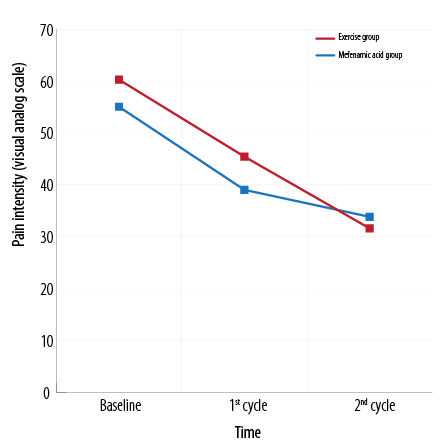
Figure 1: Change in pain intensity in the mefenamic acid and exercise group over the study period.
Today, non-medical approaches concentrate on treating dysmenorrhea. Exercise and physical activity have been suggested as alternative methods to treat dysmenorrhea and its symptoms.3,18 In the existing research, the impact of exercise was compared to using mefenamic acid as a common treatment for dysmenorrhea. Our results indicated that although pain decreased in the first month after starting the intervention in both groups, the mean pain in the exercise group was significantly more than the mefenamic acid group. In the second month, the decline in the intensity of pain in both groups continued and the mean pain in the exercise group was lower than the mefenamic acid group. The mean difference of reduced pain in the first cycle was not significant between the groups, but pain in the second cycle significantly decreased in the exercise group compared to the first cycle and baseline levels. At baseline, only moderate and severe dysmenorrhea were observed in the groups, and after one menstrual cycle, some cases of mild dysmenorrhea appeared and severe dysmenorrhea cases declined in both groups. After two cycles, no severe dysmenorrhea case was reported in both groups.
According to our results, at first, exercise is effective in reducing the pain of dysmenorrhea similar to mefenamic acid and the effect of exercise on relieving pain increases over time. Thus, it is possible that if exercise was continued on a regular basis, the pain intensity difference in the exercise group would become significant compared to the mefenamic acid group. Abbaspour et al,7 observed that exercise decreased the intensity of dysmenorrhea and sedative use after three and four cycles in high-school students. Shahr-jerdy et al,6 using the same exercise protocol reported the reduction of pain intensity and sedative use after eight weeks. Another study reported that after 12 weeks of water exercise, the duration and intensity of pain dropped.19 When comparing the impact of using a warm water bottle and exercise, researchers reported that pain intensity decreased significantly in the third month after the intervention.20 Based on these findings, it seems that the effect of exercise on dysmenorrhea appears over time.
Other studies reported improved dysmenorrhea following long-term exercise, and in those who performed regular exercise compared to those who occasionally did. In a study of athletes, the authors concluded that exercising before menarche was accompanied with higher reductions in the prevalence of dysmenorrhea, and vigorous exercise was associated with a decrease in intensity.21 In other research, women with no primary dysmenorrhea performed more than one hour of physical exercise daily compared with women who had primary dysmenorrhea.22 A three-year survey found that the prevalence of dysmenorrhea was 39% in the exercise group and 61% in the control group.23 Ganon et al,24 also reported that the duration of exercise had a significant correlation with reduced
premenstrual symptoms.
In addition to duration, the type and intensity of exercise can affect the results. Some investigators found that moderate physical activity was effective in decreasing physical symptoms associated with menstruation (e.g., pain) while others observed vigorous exercise to be effective. 18,25,26 Yet, several other researchers found a poor correlation or no relationship between physical activity and dysmenorrhea.18,27–31 A previous study in a group of women who performed vigorous exercise reported a 30% increase in dysmenorrhea cases.32 A Cochran review suggested that the different results seen can be due to the use of various exercise protocols or studies with methodological weaknesses.3 Additionally, the difference in dysmenorrhea type may influence
the results.
The exercise analgesic effect is thought to be applied through nonspecific mechanisms.6 Dysmenorrhea has a dose-response association with stress.6,23,33,34 Stress accelerates uterine contractions and menstrual pain.34,35 As a result, by decreasing stress and psychological pressures, and enhancing mood, exercise may decrease pain.8,32,36 Moreover, exercise can cause the release of endorphins, which are pain-relieving factors.8,26,37 Another potential mechanism is the improvement of pelvic blood circulation and local metabolism during exercise.8,21 Consequently, exercise may prevent prostaglandin accumulation, which results in uterine contraction, ischemia, and pain.38 Some believe that stretching exercises can be effective in removing abdominal spasms that stimulate nerve routes.4,18,39
In this study, pain duration in the second cycle in the exercise group was less than the mefenamic acid group, though the difference was not significant. Several other researchers reported the effect of exercise on reducing the duration of pain.6,7,37,40 This influence may be due to the faster transfer of prostaglandins following physical exercise.40 The effect of mefenamic acid on decreasing the length of menstruation was significantly higher than exercise in the first cycle. A larger number of students in this group reported reduced bleeding compared to the exercise group. In the second cycle, the difference in menstrual duration between the two groups was not significant, and a similar number in both groups reported reduced bleeding. Abbaspour et al7 also reported reduction in bleeding duration in the fourth cycle. In another study, by the eighth week of isometric exercise, despite a decrease in the intensity and duration of pain, there was no change in bleeding volume.37 However, by conducting a combination of stretching and resistance exercises for 90 minutes, three times weekly for eight weeks, another study reported reduced bleeding, and pain and duration.40 Therefore, the effect of exercise on duration and bleeding volume may be increased with prolonged exercise protocols or exercise type change. It seems that this effect is the result of hormonal changes.40 The prolonged menstrual intervals in the exercise group compared to the mefenamic acid group may be related to these hormonal changes. Only one study reported the effect of exercise on the length of menstrual cycle, which was not significantly different to the control group. More studies are necessary to understand
this variable.6
The number of students who needed to take analgesic drugs declined in the exercise group, which was consistent with other studies.6,7,40 Because the reduction of pain intensity increased over time in the exercise group, a decrease in taking medication is reasonable. In addition, following the reduction of pain, a decline in university absence was seen.
The limitation of our study was that the exercise protocol was only performed for a short time, and did not allow us to observe the effect of long-term exercise on dysmenorrhea. Furthermore, the results were not compared with a control group, blinding was not performed, and bleeding volume, stress level and weight changes were not assessed.
Conclusion
Regular exercise can be useful as an easy, accessible, and inexpensive approach to improve dysmenorrhea; however, the quality, intensity, and duration of exercise can influence the results. Consequently, comparative and blind studies in this field are recommended to distinguish the physical and psychological effects of exercise on dysmenorrhea.
Disclosure
The authors declared no conflicts of interest. The study was funded by the vice-chancellor for research of Mazandaran University of Medical Sciences (grant number: H-92-24).
Acknowledgements
We would like to thank all the students who participated in this study.
references
- Lefebvre G, Pinsonneault O, Antao V, Black A, Burnett M, Feldman K, et al; SOGC. Primary dysmenorrhea consensus guideline. J Obstet Gynaecol Can 2005 Dec;27(12):1117-1146.
- Marjoribanks J, Proctor M, Farquhar C, Derks RS. Nonsteroidal anti-inflammatory drugs for dysmenorrhoea. Cochrane Database Syst Rev 2010 Jan;1(1):CD001751.
- Brown J, Brown S. Exercise for dysmenorrhoea. Cochrane Database Syst Rev 2010 Feb;2(2):CD004142.
- Dawood MY. Primary dysmenorrhea: advances in pathogenesis and management. Obstet Gynecol 2006 Aug;108(2):428-441.
- Harlow SD, Campbell OM. Epidemiology of menstrual disorders in developing countries: a systematic review. BJOG 2004 Jan;111(1):6-16.
- Shahr-jerdy S, Hosseini RS, Gh ME. Effects of stretching exercises on primary dysmenorrhea in adolescent girls. Biomedical Human Kinetics 2012;4:127-132.
- Abbaspour Z, Rostami M, Najjar S. The effect of exercise on primary dysmenorrhea. J Res Health Sci 2006;6(1):26-31.
- Cheng HF, Lin YH. Selection and efficacy of self-management strategies for dysmenorrhea in young Taiwanese women. J Clin Nurs 2011 Apr;20(7-8):1018-1025.
- Collins Sharp BA, Taylor DL, Thomas KK, Killeen MB, Dawood MY. Cyclic perimenstrual pain and discomfort: the scientific basis for practice. J Obstet Gynecol Neonatal Nurs 2002 Nov-Dec;31(6):637-649.
- Al-Shidhani A, Al-Rawahi N, Al-Rawahi A, Murthi S; Sathiya Murthi P. Non-steroidal anti-inflammatory drugs (NSAIDs) use in primary health care centers in A’Seeb, Muscat: A Clinical Audit. Oman Med J 2015 Sep;30(5):366-371.
- Teimoori B, Ghasemi M, Hoseini ZS, Razavi M. The efficacy of zinc administration in the treatment of primary dysmenorrhea. Oman Med J 2016 Mar;31(2):107-111.
- Al-Raaie F, Banodkar DD. Epidemiological study of cutaneous adverse drug reactions in Oman. Oman Med J 2008 Jan;23(1):21-27.
- Al-Saeed A. Gastrointestinal and cardiovascular risk of nonsteroidal anti-inflammatory drugs. Oman Med J 2011 Nov;26(6):385-391.
- Shi S, Klotz U. Clinical use and pharmacological properties of selective COX-2 inhibitors. Eur J Clin Pharmacol 2008 Mar;64(3):233-252.
- Ozgoli G, Goli M, Moattar F. Comparison of effects of ginger, mefenamic acid, and ibuprofen on pain in women with primary dysmenorrhea. J Altern Complement Med 2009 Feb;15(2):129-132.
- Berek J, Novak E. Berek & Novak Gynocology. Lippncott Williams & Wilkins. 15th ed. Phiadelphia; 2012.
- Abdul-Razzak KK, Ayoub NM, Abu-Taleb AA, Obeidat BA. Influence of dietary intake of dairy products on dysmenorrhea. J Obstet Gynaecol Res 2010 Apr;36(2):377-383.
- Blakey H, Chisholm C, Dear F, Harris B, Hartwell R, Daley AJ, et al. Is exercise associated with primary dysmenorrhoea in young women? BJOG 2010 Jan;117(2):222-224.
- Rezvani S, Taghian F, Valiani M. The effect of aquatic exercises on primary dysmenorrhoea in nonathlete girls. Iran J Nurs Midwifery Res 2013 Sep;18(5):378-383.
- Chaudhuri A, Singh A, Dhaliwal L. A randomised controlled trial of exercise and hot water bottle in the management of dysmenorrhoea in school girls of Chandigarh, India. Indian J Physiol Pharmacol 2013 Apr-Jun;57(2):114-122.
- Izzo A, Labriola D. Dysmenorrhoea and sports activities in adolescents. Clin Exp Obstet Gynecol 1991;18(2):109-116.
- Maruf FA, Ezenwafor NV, Moroof SO, Adeniyi AF, Okoye EC. Physical activity level and adiposity: are they associated with primary dysmenorrhea in school adolescents? Afr J Reprod Health 2013 Dec;17(4):167-174.
- Golomb LM, Solidum AA, Warren MP. Primary dysmenorrhea and physical activity. Med Sci Sports Exerc 1998 Jun;30(6):906-909.
- Gannon L, Luchetta T, Pardie L, Rhodes K. Perimenstrual symptoms: relationships with chronic stress and selected lifestyle variables. Behav Med 1989;15(4):149-159.
- Choi PY, Salmon P. Symptom changes across the menstrual cycle in competitive sportswomen, exercisers and sedentary women. Br J Clin Psychol 1995 Sep;34(Pt 3):447-460.
- Israel RG, Sutton M, O’Brien KF. Effects of aerobic training on primary dysmenorrhea symptomatology in college females. J Am Coll Health 1985 Jun;33(6):241-244.
- Latthe P, Mignini L, Gray R, Hills R, Khan K. Factors predisposing women to chronic pelvic pain: systematic review. BMJ 2006 Apr;332(7544):749-755.
- Daley AJ. Exercise and primary dysmenorrhoea : a comprehensive and critical review of the literature. Sports Med 2008;38(8):659-670.
- Gordley LB, Lemasters G, Simpson SR, Yiin JH. Menstrual disorders and occupational, stress, and racial factors among military personnel. J Occup Environ Med 2000 Sep;42(9):871-881.
- Harlow SD, Park M. A longitudinal study of risk factors for the occurrence, duration and severity of menstrual cramps in a cohort of college women. Br J Obstet Gynaecol 1996 Nov;103(11):1134-1142.
- Jarrett M, Heitkemper MM, Shaver JF. Symptoms and self-care strategies in women with and without dysmenorrhea. Health Care Women Int 1995 Mar-Apr;16(2):167-178.
- Metheny WP, Smith RP. The relationship among exercise, stress, and primary dysmenorrhea. J Behav Med 1989 Dec;12(6):569-586.
- Alonso C, Coe CL. Disruptions of social relationships accentuate the association between emotional distress and menstrual pain in young women. Health Psychol 2001 Nov;20(6):411-416.
- Wang L, Wang X, Wang W, Chen C, Ronnennberg AG, Guang W, et al. Stress and dysmenorrhoea: a population based prospective study. Occup Environ Med 2004 Dec;61(12):1021-1026.
- Gannon L. The potential role of exercise in the alleviation of menstrual disorders and menopausal symptoms: a theoretical synthesis of recent research. Women Health 1988;14(2):105-127.
- Locke RJ, Warren MP. Curbside Consult: What is the effect of exercise on primary dysmenorrhea? West J Med 1999 Oct;171(4):264-265.
- Shavandi N, Taghian F, Soltani V. The effect of isometric exercise on primary dysmenorrhea. Arak Medical University Journal 2010;13(1):71-77.
- Bolton PJ, Del Mar C, O’Connor V, Dean LM, Jarrett MS. Exercise for primary dysmenorrhoea. Cochrane Libr 2012.
- Daley A. The role of exercise in the treatment of menstrual disorders: the evidence. Br J Gen Pract 2009 Apr;59(561):241-242.
- Mahvash N, Eidy A, Mehdi K, Zahra MT, Mani M, Shahla H . The effect of physical activity on primary dysmenorrhea of female university students. World Appl Sci J 2012;17(10):1246-1252.