Despite the high prevalence of tuberculosis (TB) in developing countries, primary pancreatic TB is a rare entity. TB in pancreas may present as acute or chronic pancreatitis, pancreatic abscesses, and as discrete pancreatic mass mimicking malignancy, thus making it a diagnostic challenge.1
We present a case of pancreatic TB in an immunocompetent patient with marked elevation of serum cancer antigen (CA) 19-9 levels mimicking pancreatic carcinoma clinically and radiologically.
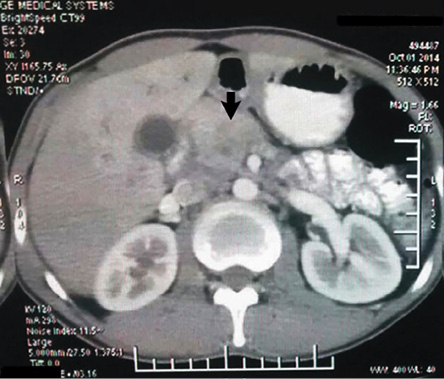
Figure 1: Contrast-enhanced computed tomography of the abdomen showing a homogenously enhancing mass lesion in the head of the pancreas.
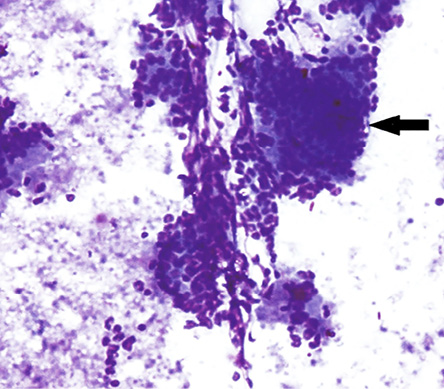
Figure 2: Endoscopic ultrasound with fine needle aspiration showing few atypical cells admixed with benign ductal epithelial cells in a necrotic background. Magnification = 200 ×.
Case Report
A 25-year-old male presented with a history of pain in the upper abdomen unrelated to meals, fever, anorexia, and weight loss. There was no history of pulmonary TB. Physical examination revealed mild epigastric tenderness. No abnormality was detected on routine laboratory investigations. Liver function tests showed: bilirubin 1.04 g/dl, alkaline phosphatase 45 IU/mL (reference range: 30−85 IU/mL), aspartate aminotransferase (AST) 25 IU/L (reference range: 8−46 IU/L), and alanine aminotransferase (ALT) 37 IU/L (reference range: 0−50 IU/L). Hepatitis and HIV serology were negative. CA 19-9 was markedly elevated (18 860 U/mL; reference range = 0−70 U/mL). Abdominal ultrasound revealed a mass lesion in the pancreatic head and contrast-enhanced computed tomography (CECT) showed an ill-defined homogeneous mass lesion with necrosis [Figure 1]. Peripancreatic lymphadenopathy was also noted. No other abnormality was detected.
The patient was referred for endo-ultrasonography and fine-needle aspiration cytology (FNAC). An endoscopic ultrasound FNAC from the pancreatic growth showed few atypical cells with large areas of necrosis [Figure 2]. In light of the raised serum CA 19-9 levels and a mass lesion in the pancreatic head on radiological examination, a possibility of adenocarcinoma could not be ruled out on FNAC, and a Whipple procedure (pancreaticoduodenectomy) was performed. Intraoperatively, a 2.5 × 2.5 cm bulky mass was found in the pancreatic head.
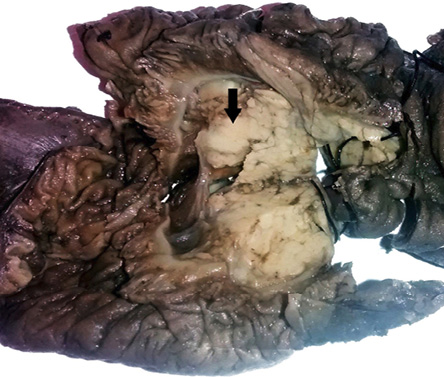
Figure 3: Whipple procedure specimen showing an ill-defined grey-white area in the head of the pancreas.
On gross examination, an ill-defined grey-white area measuring 2.5 × 2.5 × 2.0 cm with areas of necrosis was identified in the head of the pancreas [Figure 3]. The rest of the pancreas and duodenal mucosa were unremarkable. Microscopic examination showed numerous epithelioid cell granulomas with Langhans giant cells and large areas of caseation necrosis in the pancreas. The adjoining pancreatic tissue revealed features of chronic pancreatitis [Figure 4 and 5]. The entire grey-white area in the pancreas was sectioned and examined, revealing a similar picture. No evidence of malignancy was seen in multiple sections examined. Isolated peripancreatic lymph nodes also showed epithelioid cell granulomas with caseous necrosis. Ziehl Neelsen staining revealed the presence of acid-fast bacilli in the necrotic areas [Figure 5, inset].
A final diagnosis of granulomatous inflammation compatible with TB was rendered, and the patient was started on anti-tubercular therapy.
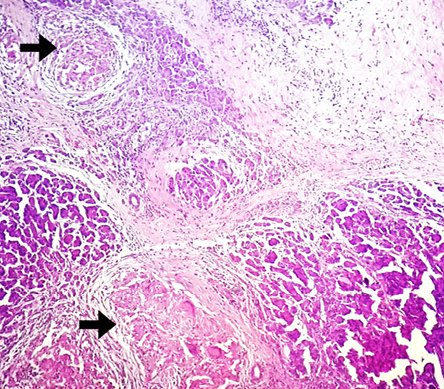
Figure 4: Hematoxylin and eosin staining showing epithelioid cell granulomas in the head of the pancreas. Magnification = 100 ×.
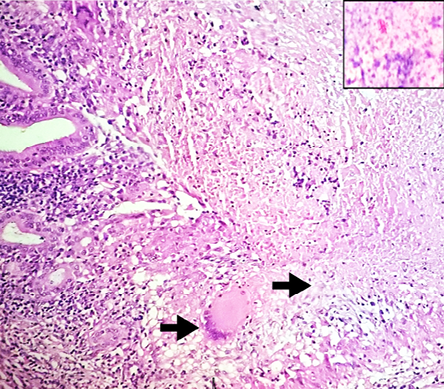
Figure 5: Hematoxylin and eosin staining showing epithelioid cell granuloma with extensive central necrosis, magnification = 400 ×. Inset showing acid-fast bacilli (AFB) in necrotic areas (Ziehl Neelsen stain. Magnification = 1000 ×).
Discussion
TB is an infectious disease caused by Mycobacterium tuberculosis. In 2013, an estimated 9.0 million people developed TB, and 1.5 million died from the disease, 360 000 of whom were HIV-positive.1 Extrapulmonary TB (EPTB) accounts for approximately 20% of all TB cases in immunocompetent patients and increases to almost 50% in patients concomitantly infected with HIV.2 The most common site for EPTB is the lymph nodes, and the abdomen is the sixth most common. The infection may occur anywhere in the gastrointestinal tract, the most common site being the ileocecal region.
The involvement of pancreas is usually seen in patients with an immunocompromised state, often in the setting of miliary TB. Isolated pancreatic TB is extremely uncommon with an incidence of less than 5%.1,2 Bhansali et al,3 reported 300 abdominal TB cases over a 12-year-period but found no cases of pancreatic TB. Although the pancreas remains protected from infection by the TB bacterium because of the presence of pancreatic enzymes, infection can occur by direct extension via the lymphatic system or hematogenous spread, or by reactivation of previous TB infection.4,5
The diagnosis of pancreatic TB often proves to be extremely challenging because of vague clinical symptoms and misleading imaging findings. The presentation is slow and insidious, with nonspecific signs and symptoms of epigastric pain, fever, anorexia and weight loss, obstructive jaundice, and sometimes a mass lesion that may be clinically indistinguishable from a neoplasm.6,7
In a study by Cho,8 pancreatic TB was found to be located in the head, tail, and body of the pancreas in decreasing frequency. CECT may show homogenously enhancing hypodense lesions with irregular borders as seen in our case. Diffusely enlarged pancreas or enlarged peripancreatic lymph nodes may also be seen. While the pancreatic duct and common bile duct are usually dilated in centrally located adenocarcinomas of the pancreatic head, they have been reported to be normal in patients with pancreatic TB.7
Apart from its role in providing high-resolution imaging, endoscopic ultrasound also provides tissue for cytopathologic examination, culture, and molecular diagnosis.7 In our case, FNAC revealed large areas of necrosis with few atypical cells thus raising the suspicion of malignancy. The features suggestive of a diagnosis of TB are the presence of caseating granulomas on histopathologic examination and acid-fast bacilli on Ziehl Neelsen stain or culture.9 The sensitivity of Ziehl Neelsen staining is about 50% compared to culture, which may take longer to perform but is more sensitive (77%). The polymerase chain reaction provides a sensitivity similar to culture, and the results can be obtained within a day.4 Treatment of pancreatic TB consists of antitubercular treatment (ATT) for at least six months.4
Serum tumor markers are used to assess the treatment response, detect relapse and, in some cases, to establish the diagnosis of malignancy. CA 19-9 is one such marker used in cancers of the gastrointestinal tract, such as those of the pancreas, biliary tract, colon, esophagus, and liver. With an upper limit of 37 U/mL, the assay's sensitivity is approximately 80% and specificity is 90%. Since the specificity approaches 100% at levels more than 1000 U/mL, the serum level of CA 19-9 has become the most useful test in the diagnosis and management of patients with pancreatic carcinoma.10 Mild elevations may be seen in several benign conditions including pancreatitis, pancreatic cysts, cholangitis, and bronchiectasis. Conditions that cause liver and kidney malfunction (such as chronic hepatitis and chronic glomerulonephritis), which metabolize CA 19-9, may also lead to elevated serum levels.11
To the best of our knowledge, our paper is the first to describe a patient with pancreatic TB and this extreme elevation of serum CA 19-9 up to levels suggestive of pancreatic adenocarcinoma (> 18000 IU/L) and in whom no malignancy was found. Such high levels of CA 19-9 along with a CT impression of pancreatic mass and suspicious cytology led to the clinical diagnosis of pancreatic adenocarcinoma, which warranted a Whipple procedure.
Conclusion
This unusual case emphasizes that TB should be included in the differential diagnosis of pancreatic masses in developing countries. Although serum CA 19-9 is the most sensitive and specific marker currently used in the diagnosis of pancreatic cancer, it may occasionally show markedly elevated values in non-neoplastic diseases such as TB as seen in our case. Appropriate and timely diagnosis along with antitubercular therapy is advised to avoid unnecessary surgical intervention, especially in young patients.
Disclosure
The authors declared no conflicts of interest.
references
- 1. Raghawan P, Rajan D. Isolated Pancreatic Tuberculosis mimicking malignancy in an immunocompetent host. Case Rep Med. 2012;2012:501246.
- 2. Sharma SK, Mohan A. Extrapulmonary tuberculosis. Indian J Med Res 2004 Oct;120(4):316-353.
- 3. Bhansali SK. Abdominal tuberculosis. Experiences with 300 cases. Am J Gastroenterol 1977 Apr;67(4):324-337.
- 4. Singh DK, Haider A, Tatke M, Kumar P, Mishra PK. Primary pancreatic tuberculosis masquerading as a pancreatic tumor leading to Whipple’s pancreaticoduodenectomy. A case report and review of the literature. JOP 2009;10(4):451-456.
- 5. Franco-Paredes C, Leonard M, Jurado R, Blumberg HM, Smith RM. Tuberculosis of the pancreas: report of two cases and review of the literature. Am J Med Sci 2002 Jan;323(1):54-58.
- 6. Chen CH, Yang CC, Yeh YH, Yang JC, Chou DA. Pancreatic tuberculosis with obstructive jaundice–a case report. Am J Gastroenterol 1999 Sep;94(9):2534-2536.
- 7. De Backer AI, Mortelé KJ, Bomans P, De Keulenaer BL, Vanschoubroeck IJ, Kockx MM. Tuberculosis of the pancreas: MRI features. AJR Am J Roentgenol 2005 Jan;184(1):50-54.
- 8. Cho SB. Pancreatic tuberculosis presenting with pancreatic cystic tumor: a case report and review of the literature. Korean J Gastroenterol 2009 May;53(5):324-328.
- 9. Tauro LF, Martis JS, George C, Kamath A, Lobo G, Hegde BR. Tuberculous mastitis presenting as breast abscess. Oman Med J 2011 Jan;26(1):53-55.
- 10. Steinberg W. The clinical utility of the CA 19-9 tumor-associated antigen. Am J Gastroenterol 1990 Apr;85(4):350-355.
- 11. van der Veek PP, de Vos Tot Nederveen Cappel WH, Langers AM, van Hoek B. Two Patients with Extremely Elevated Tumor Markers: Where Is the Malignancy? Gastroenterol Res Pract. 2011;2011:123743.