Mucormycosis is a rare and opportunistic fungal infection caused by the order Mucorales. Common sites of infection include rhino-orbito-cerebral, pulmonary, and cutaneous, with gastric mucosa being the rarest location.1 Symptoms of gastrointestinal (GI) mucormycosis vary and range from fever, nausea, vomiting, abdominal pain, GI bleeding, to perforation. Definitive diagnosis depends on the histopathological demonstration of fungal hyphae typical of mucormycetes in affected tissue biopsies. Tissue culture is considered essential for diagnosis and treatment, aiding in identifying sensitive antifungal medications by determining the genus and species of the fungus.2
Mucormycosis is a dangerous infection requiring aggressive treatment. Medical management includes supportive care, elimination of predisposing factors, and antifungal medications such as liposomal amphotericin B, isavuconazole, or posaconazole. Surgical debridement of devitalized necrotic tissue and debulking of infection are frequently necessary.3
The onset of COVID-19 has led to the emergence of rare opportunistic co-infections, with India reporting the largest number of cases, most commonly rhino-orbital.4 This association is attributed to infection-related factors and treatment-related factors.
Gastric mucormycosis, a lethal fungal infection caused by Mucorales invasion into gastric tissues, is a very rare condition, accounting for 7% of all reported cases, with the stomach being the most commonly affected organ (67%). Mortality rates in immunocompromised patients exceed 50%.5
We report a case of a patient with COVID-19 pneumonia developing gastric mucormycosis.
Case Report
In April 2021, a 66-year-old female presented to our institute with shortness of breath, malaise, and sore throat. Upon presentation, she was drowsy and hypoxemic, with a respiratory rate of 24 breaths/minute and oxygen saturation of 55% on room air, a pulse rate of 84 beats/min, and blood pressure of 140/69 mmHg. After receiving 15 L/min of oxygen via a non-rebreather mask, her oxygen saturation improved to 92%. Her laboratory workup showed raised inflammatory markers (white cell count of 13.81 × 109/L, absolute neutrophil count of 10.6 × 109/L, and C-reactive protein of 269 mg/L), acute kidney injury (creatinine of 608 µ/L, potassium of 6.7 mmol/L, sodium of 126 mmol/L, urea of 23.1 mmol/L), normal hemoglobin (11 g/dL) and troponin T 28.4 ng/L, and liver function tests were unremarkable (alanine aminotransferaseof 34 IU/L, aspartate aminotransferase of 49 IU/L, and alkaline phosphataseof 185 IU/L). A semi-erect anteroposterior portable chest X-ray revealed bilateral infiltrates suggestive of COVID-19 pneumonia [Figure 1]. The patient tested positive for COVID-19 and was started on intravenous dexamethasone, ceftriaxone, frusemide, and gentle hydration. She was admitted with the impression of acute respiratory distress syndrome secondary to COVID-19.
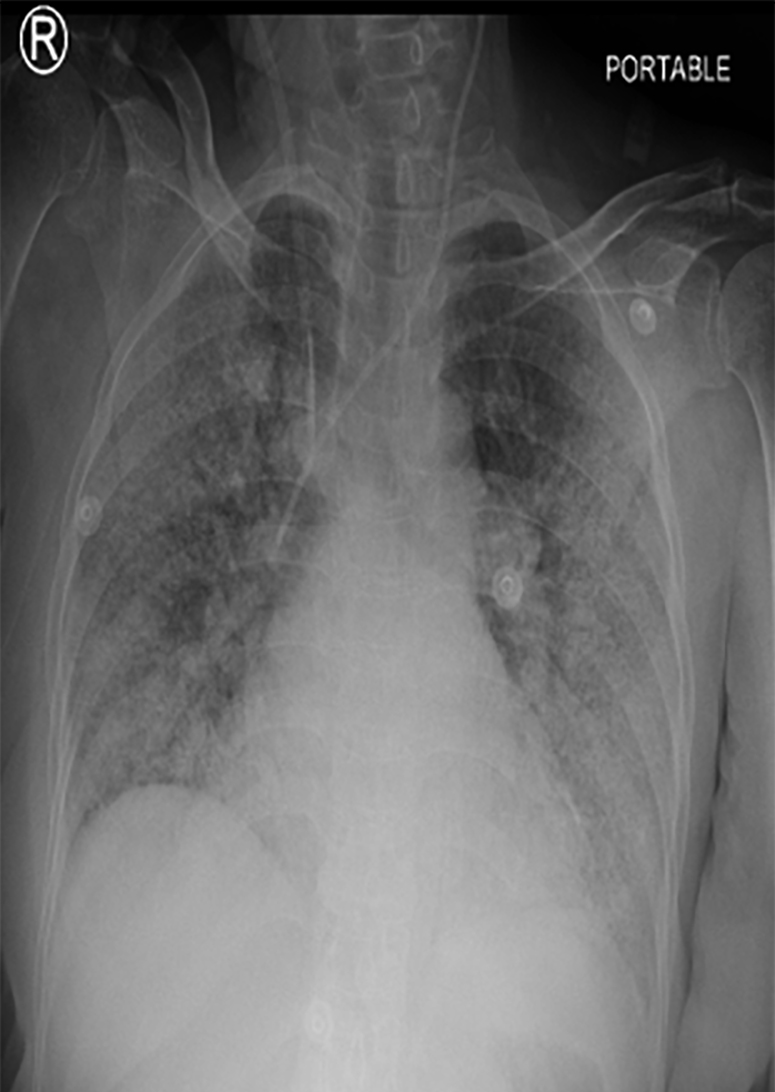 Figure 1: A semi-erect anteroposterior portable chest X-Ray showing bilateral infiltrates, suggestive of COVID-19 pneumonia.
Figure 1: A semi-erect anteroposterior portable chest X-Ray showing bilateral infiltrates, suggestive of COVID-19 pneumonia.
The patient remained in need of aggressive respiratory support, leading to intubation and transfer to the intensive care unit (ICU).
In the COVID-19 ICU, the patient received ceftriaxone, azithromycin, and dexamethasone 10 mg BID. Anticoagulation with low-molecular weight heparin (5000iu BID) was administered. She showed steady recovery throughout her ICU admission. On day nine, coffee-ground material was aspirated through her nasogastric tube despite being on a proton pump inhibitor, so the dose was increased. Her hemoglobin dropped to 8.4 g/dL. On day 11, she was extubated and shifted to the general ward. On the following day, she was again found to have coffee-ground nasogastric aspirate with a continuous drop in her hemoglobin to 6.8 g/dL and received two units of packed red blood cells. A subsequent abdominal computed tomography (CT) scan revealed significant thickening of the stomach wall with specks of air, suggesting a possible sealed perforation or emphysematous gastritis [Figure 2]. As a result, steroids were stopped and her antibiotics were upgraded with the impression of hollow viscous perforation. This was followed by an upper GI endoscopy that showed antral ulcer suspicious for malignancy, for which a biopsy was taken.
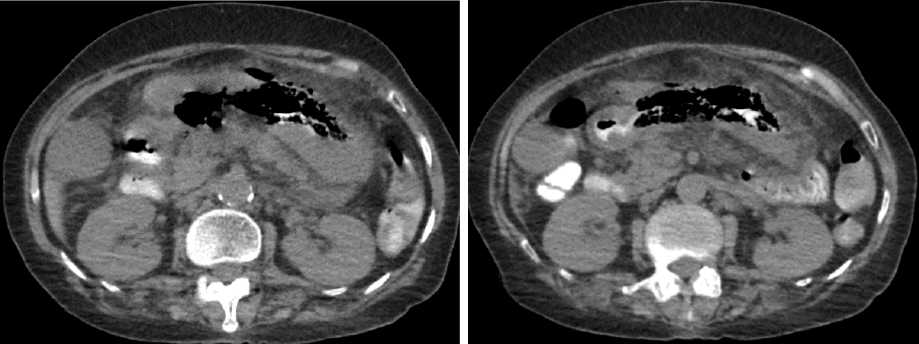 Figure 2: A CT showing a significant thickening of stomach wall with specks of air along the stomach, suggestive of a possible sealed perforation or emphysematous gastritis.
Figure 2: A CT showing a significant thickening of stomach wall with specks of air along the stomach, suggestive of a possible sealed perforation or emphysematous gastritis.
Following these findings, the patient was referred to the general surgery. Surgical review found tenderness in the epigastrium and right iliac fossa but no signs of peritonitis. Impression was sealed perforation due to an inflammatory/neoplastic gastric ulcer. The patient was treated conservatively with nil per os, a nasogastric tube, antibiotics, and close monitoring. She was on meropenem and vancomycin, which were guided by previous positive blood cultures since her ICU admission. Five days later, the gastric biopsy revealed gastric mucosa with extensive ulceration. Many fragments of necrotic slough contain abundant fungal hyphae morphology consistent with mucormycosis. The fungal elements show numerous broad, irregular, non-septate, and pauciseptate ribbon-like hyphae branching at 90-degree angles, suggestive of mucormycosis, and angioinvasion was present. There was no evidence of malignancy [Figure 3]. She was started on liposomal amphotericin B while continuing other antibiotics. A non-contrast enhanced CT scan of the brain was done to rule out rhinocerebral mucormycosis and was found to be unremarkable.
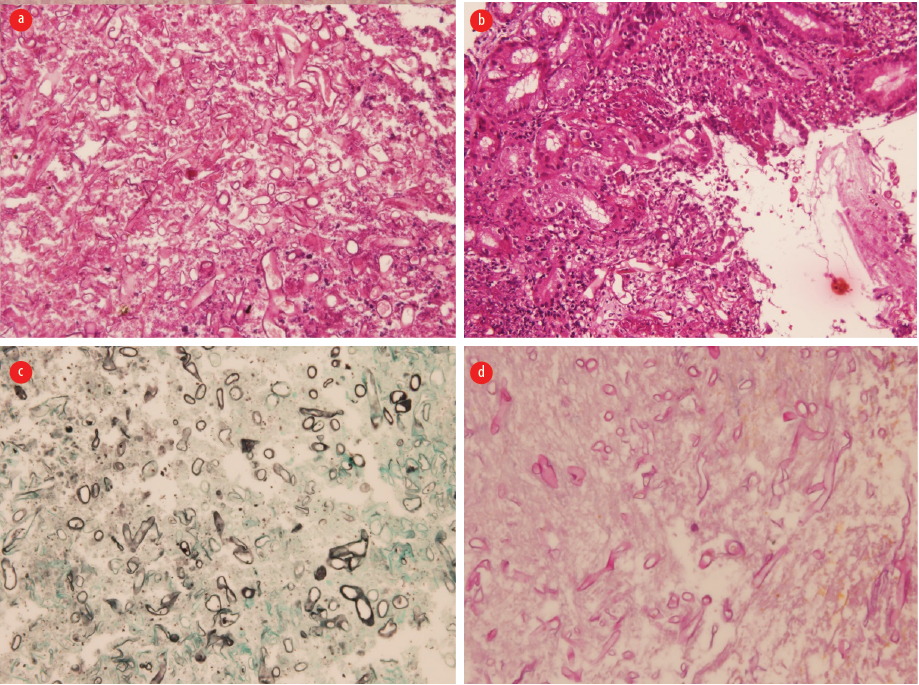 Figure 3: (a) Hematoxylin and eosin section showed gastric mucosa with ulceration and necroinflammatory debris, fungal hyphae present, magnification = 100 ×. (b) Hematoxylin and eosin section showed numerous broad-based irregular pauciseptate ribbon-like fungal hyphae consistent with mucormycosis, magnification = 100 ×. (c) Grocott methenamine silver stain highlights the fungal hyphae, magnification = 200 ×.
Figure 3: (a) Hematoxylin and eosin section showed gastric mucosa with ulceration and necroinflammatory debris, fungal hyphae present, magnification = 100 ×. (b) Hematoxylin and eosin section showed numerous broad-based irregular pauciseptate ribbon-like fungal hyphae consistent with mucormycosis, magnification = 100 ×. (c) Grocott methenamine silver stain highlights the fungal hyphae, magnification = 200 ×.
(d) Periodic acid-Schiff stain highlights the fungal hyphae, magnification = 200 ×.
On day 20, her condition worsened with white cell count rising to 24 × 109/L, absolute neutrophil count increasing to 22.9 × 109/L, and platelets dropping to 80 × 106/L. A repeat abdominal CT scan showed increased free fluid in the perihepatic area, likely infected, increased abdominal fat stranding, posterior stomach wall showing increased thickness, anterior gastric emphysema, as well as findings of COVID-19 pneumonia [Figure 4]. Despite counseling for surgical intervention, the family refused, and the patient was moved back to the ICU. Her condition deteriorated, leading to a decision not to resuscitate. On day 30, the patient passed away due to ongoing severe septic shock and multi-organ failure. Informed consent was obtained from the patient’s son.
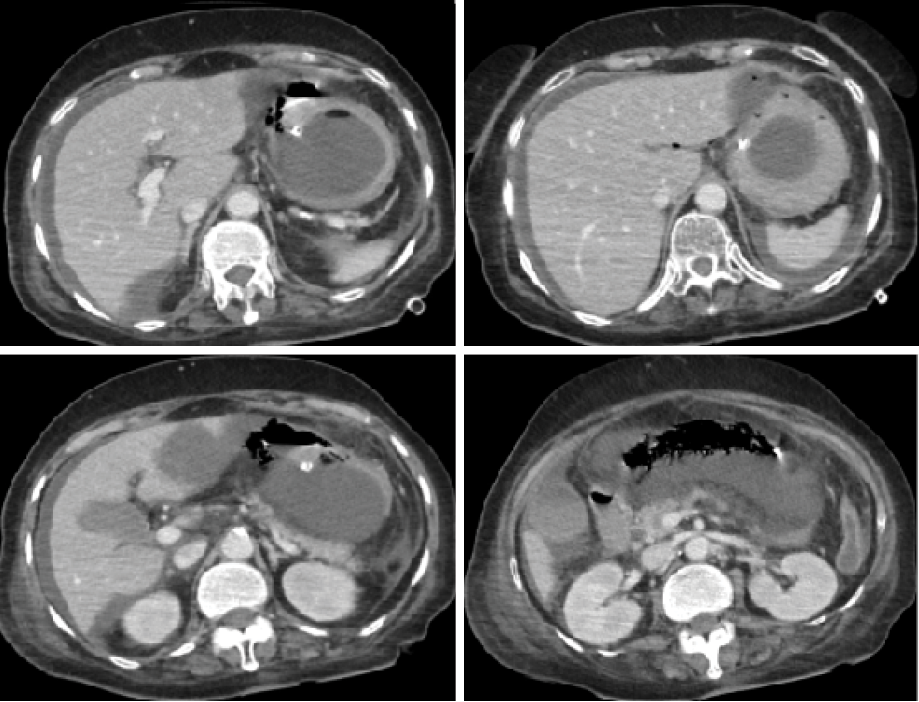 Figure 4: A CT showing significant free fluid in the perihepatic area with increased abdominal fat stranding and gastric emphysema.
Figure 4: A CT showing significant free fluid in the perihepatic area with increased abdominal fat stranding and gastric emphysema.
Discussion
Mucormycosis, or “black fungus”, is a rare and dangerous opportunistic infection caused by Mucorales. The most common organism is Rhizopus oryzae. The disease usually affects immunocompromised patients, with predisposing factors such as uncontrolled diabetes mellitus, solid organ or stem cell transplant, underlying hematological malignancy, major trauma or burns, use of steroids, iron overload conditions, severe neutropenia, and disseminated chronic infections.6
Mucormycosis can affect any organ system, but most commonly presents as rhino-cerebral, followed by pulmonary and cutaneous. Primary gastrointestinal infection is uncommon, accounting for approximately 7% of all cases, with the stomach being the most common (67%), followed by the colon (21%), small intestine (4%), and esophagus (2%).7 Mucormycosis is characterized by hyphae invading the vasculature, leading to infarction and necrosis of the host’s tissues. Symptoms of GI mucormycosis may vary but range from fever, nausea, vomiting, abdominal pain, GI bleeding, and perforation.5
The true incidence of mucormycosis remains unknown due to diagnostic challenges. The Leading International Fungal Education portal has estimated the annual incidence of acute invasive mucormycosis might be around 10 000 cases.8 The ongoing COVID-19 pandemic has seen a global increase in COVID-19-associated mucormycosis, particularly in India.9 The hypothesis is that COVID-19 provides an ideal environment for the germination of Mucorales spores, which are hypoxia, hyperglycemia (steroid-induced, new onset, or diabetes), acidosis (metabolic, diabetic), high iron levels, and decreased phagocytic activity due to immunosuppression (SARS-CoV-2 mediated, steroid-mediated, or background co-morbidities).3
Diagnosis is challenging and requires a high index of suspicion, especially in immunocompromised COVID-19-positive patients. Proper imaging and confirmatory biopsy are crucial. Treatment involves aggressive surgical debridement, antifungal medications, and supportive care. The use of steroids in COVID-19 patients should be judicious to reduce the incidence of COVID-19-associated mucormycosis.
Conclusion
Mucormycosis is an opportunistic infection and emerged as a significant concern during the COVID-19 pandemic. The hypoxia and reduced immunity associated with COVID-19 infection have allowed for this infection to thrive. The interaction between COVID-19, hypoxia, and compromised immunity creates an environment conducive to mucormycosis development. A high index of suspicion is essential for early diagnosis, enabling the prompt initiation of aggressive surgical debridement and appropriate antifungal therapy.
Disclosure
The authors declare no conflicts of interest.
references
- 1. Hibbett DS, Binder M, Bischoff JF, Blackwell M, Cannon PF, Eriksson OE, et al. A higher-level phylogenetic classification of the fungi. Mycol Res 2007 May;111(Pt 5):509-547.
- 2. Skiada A, Pavleas I, Drogari-Apiranthitou M. Epidemiology and diagnosis of mucormycosis: an update. J Fungi (Basel) 2020 Nov;6(4):265.
- 3. Singh AK, Singh R, Joshi SR, Misra A. Mucormycosis in COVID-19: a systematic review of cases reported worldwide and in India. Diabetes Metab Syndr 2021;15(4):102146.
- 4. Pal R, Singh B, Bhadada SK, Banerjee M, Bhogal RS, Hage N, et al. COVID-19-associated mucormycosis: an updated systematic review of literature. Mycoses 2021 Dec;64(12):1452-1459.
- 5. Roden MM, Zaoutis TE, Buchanan WL, Knudsen TA, Sarkisova TA, Schaufele RL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis 2005 Sep;41(5):634-653.
- 6. Ibrahim AS, Spellberg B, Walsh TJ, Kontoyiannis DP. Pathogenesis of mucormycosis. Clin Infect Dis 2012;54 Suppl 1(Suppl 1):S16-S22.
- Naqvi HA, Nadeem Yousaf M, Chaudhary FS, Mills L. Gastric Mucormycosis: An infection of fungal invasion into the gastric mucosa in immunocompromised patients. Case Rep Gastrointest Med. 2020 Sep 16;2020:8876125. doi: 10.1155/2020/8876125.
- 7. Bongomin F, Gago S, Oladele RO, Denning DW. Global and multi-national prevalence of fungal diseases-estimate precision. J Fungi (Basel) 2017;3(4):57.
- 8. Muthu V, Rudramurthy SM, Chakrabarti A, Agarwal R. Epidemiology and pathophysiology of COVID-19-associated mucormycosis: India versus the rest of the world. Mycopathologia 2021 Dec;186(6):739-754.