Asolid pseudopapillary tumor (SPT) is a rare exocrine pancreatic neoplasm, accounting for 1–2% of all exocrine pancreatic tumors.1 In 1959, Dr. Frantz described the morphological features of the SPT.2,3 SPT has many synonyms. These include solid cystic tumor, papillary cystic neoplasm, papillary cystic tumor, papillary epithelial neoplasia, solid and papillary epithelial neoplasia, papillary epithelial tumor, Frantz’s tumor, solid and papillary tumor, solid-cystic-papillary epithelial neoplasm, benign or malignant papillary tumor of the pancreas, and adenocarcinoma of the pancreas in childhood.4,5 In 1996, the World Health Organization (WHO) renamed it SPT of the pancreas.2
SPT usually has a benign course and is seen in young non-Caucasian females with the greatest incidence in the second and third decades of life3.Most patients are asymptomatic at the time of the diagnosis; however, some of them may present with a gradually enlarging abdominal mass or complain of vague abdominal pain.4,6 Here we reported our experience with an SPT in a 34-year-old pregnant woman and have included a review of the current literature.
Case Report
A 34-year-old pregnant woman was referred to our hospital with a history of an abdominal mass, which was discovered incidentally in an anomaly ultrasound (US) scan performed in a private medical center at 28 weeks gestation. The mass was described as a heterogeneous and originating from the lower pole of the left kidney. No detailed report or images were available when the patient presented to our hospital.
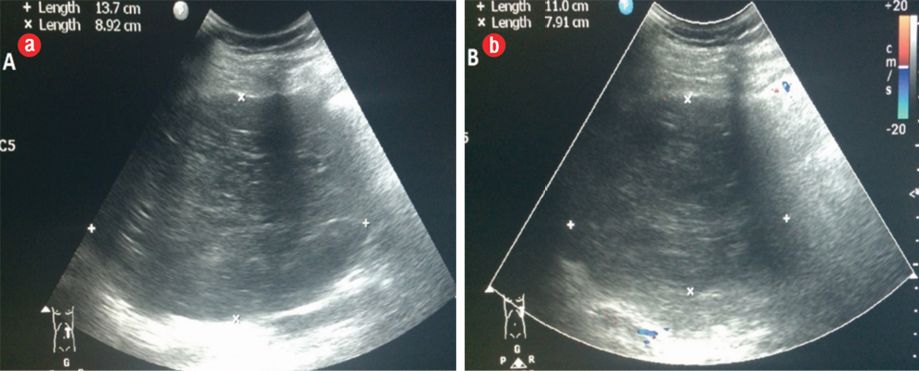
Figure 1: (a) Transverse upper abdominal ultrasonography: a hypoechoic heterogeneous mass of the pancreas. (b) Doppler ultrasonography showed no apparent tumoral vascularization.
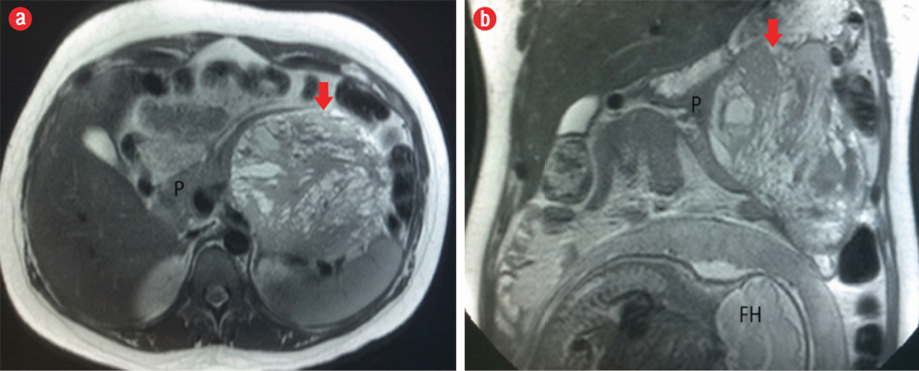
Figure 2: (a) Axial and (b) coronal T2-weighted MRI showed a well-marginated lesion (red arrow), heterogeneously hyperintense, in the body/tail of the pancreas (P) with a hypointense fibrous pseudocapsule. The fetal head (FH) can also be seen in the image.
The woman had no significant medical history, and this was her first pregnancy. On physical examination, the patient was not pale or jaundiced, and head, neck, chest, and abdominal examination were normal. Blood workup, including tumor markers, liver, and renal function tests, was unremarkable.
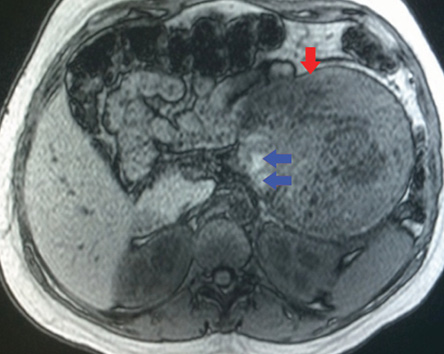
Figure 3: On an axial unenhanced fat-suppressed T1-weighted MRI the fibrous pseudocapsule was also hypointense (red arrow), and there was an internal peripheral high signal intensity rim (blue arrows), a finding consistent with hemorrhage.
Abdominal ultrasound was repeated in our hospital. It showed a well-defined heterogeneously hypoechoic mass measuring 13.7 cm × 9.0 cm in diameter. The mass was heterogeneous hypoechoic and hypovascular in color Doppler [Figure 1]. Since the patient was pregnant, she was evaluated with a limited non-contrast magnetic resonance imaging (MRI) using a 3.0 Tesla scanner. The patient gave her informed consent.
The MRI showed a well-defined mass related to the pancreatic tail and body, measuring 13.7 cm × 11.7 cm × 4.2 cm, with solid and cystic components [Figure 2]. It was of heterogeneous texture and contained multiple fluid levels of different signal intensities due to the presence of blood of different ages [Figure 3]. Other abdominal organs were normal. Based on the MRI findings, a diagnosis of solid pseudopapillary pancreatic tumor was made. Follow-up with the patient was lost.
She returned to the hospital three months later after a cesarean delivery at 38 weeks gestation. Repeated MRI revealed interval progression in the tumor size from 13.7 cm × 11.7 cm × 4.2 cm to 16.8 cm × 12.3 cm × 11.5 cm, which otherwise had the same described features as the previous MRI examination. Intravenous contrast medium was given in the second MRI, which showed enhancement of the solid component of the tumor [Figure 4]. The patient underwent surgery, and histopathology results confirmed the diagnosis of a SPT.
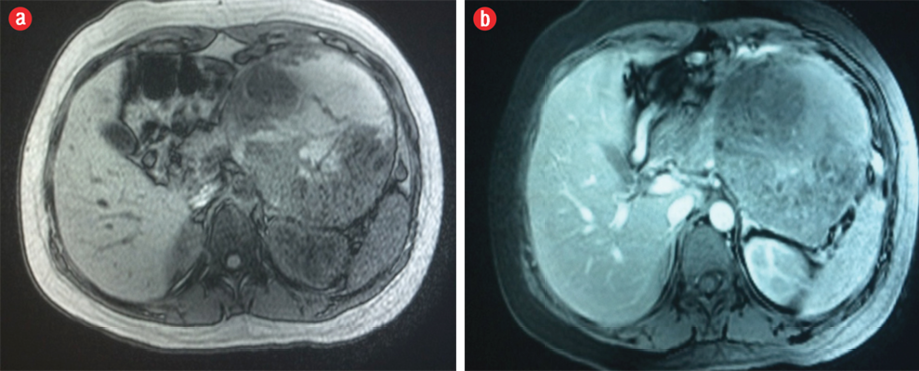
Figure 4: Axial (a) pre-contrast and (b) post-contrast fat-suppressed T1-weighted MRI showed enhancement of the solid component of the neoplasm.
Discussion
SPT is a rare pancreatic tumor, accounting for 1–2% of all exocrine pancreatic neoplasms.1 SPT is usually benign and affects young non-Caucasian females with the greatest incidence in the second and third decades of life. It has a female to male ratio of 4:1.3 Most patients are asymptomatic at the time of the diagnosis; however, some of them may present with a gradually enlarging abdominal mass or complain of vague abdominal pain.4,6 Usually, there are no abnormalities in serum markers of pancreatic neoplasm (e.g., serum amylase levels) or pancreatic cancer markers.7 Diagnosis is often incidental.7
Most SPTs have a benign clinical course; however, malignant degeneration with metastasis and invasion of adjacent structures has been reported in some cases.6 Even when aggressive, these tumors have a good prognosis with a long life expectancy.6 Multidetector computed tomography (MDCT) and MRI are very helpful in evaluating intralesional components such as presence of hemorrhage, fluid level, calcifications, septa, internal nodule and degree of enhancement.6,8 SPTs have typical and atypical imaging features.9 The typical appearance in CT is a well-encapsulated heterogeneous lesion with varying solid and cystic components attributed to hemorrhagic degeneration.10 The solid part of the tumor is usually located at the periphery and shows enhancement after contrast administration in the arterial phase, whereas, the cystic component is located centrally.11 Additionally, calcification and slow enhancement of the internal component have been reported.6 On MRI, the SPT typically appears as a well-defined pancreatic lesion with heterogeneous intensity in T1- and T2-weighted imaging owing to the complex nature of the lesion.7 Intralesional areas of high signal intensity on T1-weighted images and a low or inhomogeneous signal intensity on T2-weighted images indicate blood products within the lesion.4 Post-gadolinium administration, the lesion showed peripheral enhancement owing to the enhancing peripherally located solid part.11 Atypical SPT manifestations include peripancreatic fat invasion, invasion of the surrounding parenchyma, vessels and surrounding organs, lymph node metastases, ductal obstruction, and liver metastases.6,10,12
In our case, we found a typical cystic-solid lesion containing multiple fluid levels of different signal intensities due to the presence of blood products of different age. The lesion showed a peripherally located enhancing solid component. There are several pancreatic cystic neoplasms with a high rate of diagnostic overlap (e.g., serous cystadenomas, mucin-producing tumors, islet cell tumors) which makes the diagnosis of SPT difficult due to its heterogeneous appearance and the overlap with other cystic pancreatic neoplasms.7 However, in our case, the typical MRI features of the SPT, and the sex and age of the patient led us to the correct diagnosis and enabled us to differentiate it from the other pancreatic tumors. It is important to differentiate SPTs from ductal adenocarcinoma. Adenocarcinoma is frequently seen in male adults, and it is usually small at the time of diagnosis. Unlike ductal adenocarcinoma, SPTs are soft and seldom causes bile duct or pancreatic duct obstruction, even when it is located in the head of the pancreas.13
US- or CT-guided fine-needle aspiration (FNA) can be very helpful as a preoperative diagnostic tool.14,15 In our case, preoperative diagnostic FNA was not considered due to typical radiological features of the mass and the worrying interval progression in tumor size between the first and second MRI examinations.
Macroscopically, a SPT is usually composed of a mixture of solid, cystic, and pseudopapillary components in different proportions. The solid part consist of uniform and polygonal epithelial cells with well-vascularized stroma and a discohesive arrangement.16 Solid and pseudopapillary proliferation of homomorphous cells without increased mitoses or cytological atypia are considered the key histological features.17,18 Immunohistochemical stains are helpful in making the diagnosis. Solid pseudopapillary neoplasm cells positively stain for vimentin, progesterone receptor, β-catenin, CD56, neuron-specific enolase (NSE), CD10, cyclin D1 and, negative membranous E-cadherin.19,20 In our case, the sections from the mass showed a relatively well-encapsulated neoplasm composed of solid sheets and pseudopapillary structures of uniform polygonal cells. Foci of cystic degeneration, hemorrhage, and occasional foreign body type granulomas with cholesterol cleft were noted. The neoplastic cells show round to oval vesicular nuclei with inclusion, inconspicuous nucleoli, and a moderate amount of eosinophlic cytoplasm. Immunostains show strong positivity of the tumor cells for CD56, CD10, B-catenin, and NSE. Complete surgical resection is the treatment of choice for SPTs.21 Incomplete tumor excision should be avoided due to the risk of tumor dissemination and higher recurrence rate.22 Extensive lymphatic dissection is not recommended because SPTs have a very low incidence of lymph node metastasis (<2%).23,24
Generally, SPTs have an excellent prognosis with an overall five-year survival rate of around 95%.25 Malignant behavior is seen in approximately 10–15% of the cases, mostly metastasized to the liver or peritoneum.26 Therefore, long-term follow-up is recommended. On follow-up of our patient, clinical examination, routine laboratory tests, abdominal US and MRI did not show any evidence of tumor recurrence.
Conclusion
In conclusion, although SPT of the pancreas is rare, the presence of a relatively large, well-defined, hemorrhagic, cystic pancreatic mass in a young female patient should raise the suspicion of the presence of this tumor.
Disclosure
The authors declared no conflicts of interest.
references
- Shaikh S, Arya S, Ramadwar M, Barreto SG, Shukla PJ, Shrikhande SV. Three cases of unusual solid pseudopapillary tumors. Can radiology and histology aid decision-making? JOP 2008;9(2):150-159.
- Bostanoglu S, Otan E, Akturan S, Hamamci EO, Bostanoglu A, Gokce A, et al. Frantz’s tumor (solid pseudopapillary tumor) of the pancreas. A case report. JOP 2009;10(2):209-211.
- Vollmer CM Jr, Dixon E, Grant DR. Management of a solid pseudopapillary tumor of the pancreas with liver metastases. HPB (Oxford) 2003;5(4):264-267.
- Ganeshan DM, Paulson E, Tamm EP, Taggart MW, Balachandran A, Bhosale P. Solid pseudopapillary tumors of the pancreas: current update. Abdomen Imaging 2013;1373-1382.
- Cantisani V, Mortele KJ, Levy A, Glickman JN, Ricci P, Passariello R, et al. MR imaging features of solid pseudopapillary tumor of the pancreas in adult and pediatric patients. AJR Am J Roentgenol 2003 Aug;181(2):395-401.
- Choi JY, Kim MJ, Kim JH, Kim SH, Lim JS, Oh YT, et al. Solid pseudopapillary tumor of the pancreas: typical and atypical manifestations. AJR Am J Roentgenol 2006 Aug;187(2):W178-186.
- Coleman KM, Doherty MC, Bigler SA. Solid-pseudopapillary tumor of the pancreas. Radiographics 2003 Nov-Dec;23(6):1644-1648.
- Kalb B, Sarmiento JM, Kooby DA, Adsay NV, Martin DR. MR imaging of cystic lesions of the pancreas. Radiographics 2009 Oct;29(6):1749-1765.
- Zinner MJ, Shurbaji MS, Cameron JL. Solid and papillary epithelial neoplasms of the pancreas. Surgery 1990 Sep;108(3):475-480.
- Buetow PC, Buck JL, Pantongrag-Brown L, Beck KG, Ros PR, Adair CF. Solid and papillary epithelial neoplasm of the pancreas: imaging-pathologic correlation on 56 cases. Radiology 1996 Jun;199(3):707-711.
- Kehagias D, Smyrniotis V, Gouliamos A, Vlahos L. Cystic pancreatic neoplasms: computed tomography and magnetic resonance imaging findings. Int J Pancreatol 2000 Dec;28(3):223-230.
- Wang DB, Wang QB, Chai WM, Chen KM, Deng XX. Imaging features of solid pseudopapillary tumor of the pancreas on multi-detector row computed tomography. World J Gastroenterol 2009 Feb;15(7):829-835.
- Nakatani K, Watanabe Y, Okumura A, Nakanishi T, Nagayama M, Amoh Y, et al. MR imaging features of solid-pseudopapillary tumor of the pancreas. Magn Reson Med Sci 2007;6(2):121-126.
- Jani N, Dewitt J, Eloubeidi M, Varadarajulu S, Appalaneni V, Hoffman B, et al. Endoscopic ultrasound-guided fine-needle aspiration for diagnosis of solid pseudopapillary tumors of the pancreas: a multicenter experience. Endoscopy 2008 Mar;40(3):200-203.
- Zamboni GA, D’Onofrio M, Principe F, Pozzi Mucelli R. Focal pancreatic lesions: accuracy and complications of US-guided fine-needle aspiration cytology. Abdom Imaging 2010 Jun;35(3):362-366.
- Tang LH, Aydin H, Brennan MF, Klimstra DS. Clinically aggressive solid pseudopapillary tumors of the pancreas: a report of two cases with components of undifferentiated carcinoma and a comparative clinicopathologic analysis of 34 conventional cases. Am J Surg Pathol 2005 Apr;29(4):512-519.
- Matos JM, Grützmann R, Agaram NP, Saeger HD, Kumar HR, Lillemoe KD, et al. Solid pseudopapillary tumor neoplasms of the pancreas: A multi-institutional study of 21 patients. J Surg Res 2009;157:137-142 .
- Geers C, Moulin P, Gigot JF, Weynand B, Deprez P, Rahier J, et al. Solid and pseudopapillary tumor of the pancreas–review and new insights into pathogenesis. Am J Surg Pathol 2006 Oct;30(10):1243-1249.
- Yeh TS, Jan YY, Chiu CT, Ho YB, Chen TC, Lee KF, et al. Characterisation of oestrogen receptor, progesterone receptor, trefoil factor 1, and epidermal growth factor and its receptor in pancreatic cystic neoplasms and pancreatic ductal adenocarcinoma. Gut 2002 Nov;51(5):712-716.
- Gomez P, Yorke R, Ayala AG, Ro JY. Solid-pseudopapillary neoplasm of pancreas with long delayed liver metastasis. Ann Diagn Pathol 2012 Oct;16(5):380-384.
- Lee JS, Han HJ, Choi SB, Jung CW, Song TJ, Choi SY. Surgical outcomes of solid pseudopapillary neoplasm of the pancreas: a single institution’s experience for the last ten years. Am Surg 2012 Feb;78(2):216-219.
- Patil TB, Shrikhande SV, Kanhere HA, Saoji RR, Ramadwar MR, Shukla PJ. Solid pseudopapillary neoplasm of the pancreas: a single institution experience of 14 cases. HPB (Oxford) 2006;8(2):148-150.
- Vassos N, Agaimy A, Klein P, Hohenberger W, Croner RS. Solid-pseudopapillary neoplasm (SPN) of the pancreas: case series and literature review on an enigmatic entity. Int J Clin Exp Pathol 2013;6(6):1051-1059.
- Martin RC, Klimstra DS, Brennan MF, Conlon KC. Solid-pseudopapillary tumor of the pancreas: a surgical enigma? Ann Surg Oncol 2002 Jan-Feb;9(1):35-40.
- Papavramidis T, Papavramidis S. Solid pseudopapillary tumors of the pancreas: review of 718 patients reported in English literature. J Am Coll Surg 2005 Jun;200(6):965-972.
- Yoon DY, Hines OJ, Bilchik AJ, Lewin K, Cortina G, Reber HA. Solid and papillary epithelial neoplasms of the pancreas: aggressive resection for cure. Am Surg 2001 Dec;67(12):1195-1199.