A 36-year-old male was admitted with right-sided pleuritic chest pain that had lasted five days. There was no fever or chills, but he reported shortness of breath and long-standing coughing and wheezing. The cough was intermittently productive of brown sputum. The patient was diagnosed with bronchial asthma 10 years prior. He was managed by a pulmonologist until he lost his job and insurance three years before his presentation to the hospital. In those three years he continued to treat himself with 20 to 60mg prednisone daily to control his coughing and wheezing. His medical history was otherwise unremarkable. He was married with two healthy sons, and he had no history of smoking.
On examination, the patient was thin and in mild respiratory distress. Vital signs were as follows: temperature 37.6oC, heart rate 104beats/min (regular), blood pressure 127/76mmHg, respiratory rate 28breaths/min, and oxygen saturation 94% while on 2L/min of oxygen via a nasal cannula. Significant physical examination findings included the following: right-sided pleural rub and shallow inspirations, regular heart sounds without murmurs or gallop, and no lymphadenopathy or rash.
Pertinent laboratory findings included the following: white blood cells count 12,200/μL (79% neutrophils and 15% lymphocytes), hemoglobin level 11.8g/dL, platelet count 276,000/μL, and human immunodeficiency virus (HIV) test was negative. Liver enzymes, kidney function tests, and coagulation studies were within normal limits. Chest X-ray (CXR) on admission and computed tomography (CT) scan are shown in Figures 1 and 2.
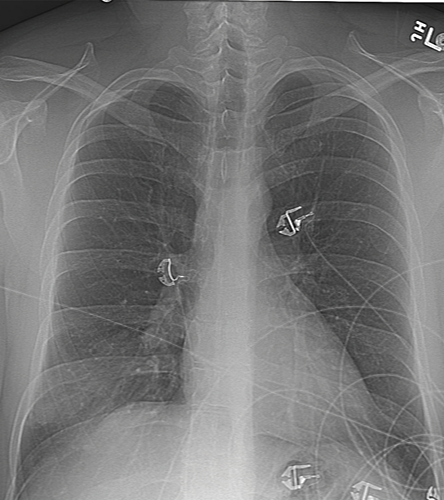
Figure 1: Chest X-ray on admission of a 36-year-old male with right-sided pleuritic chest pain.
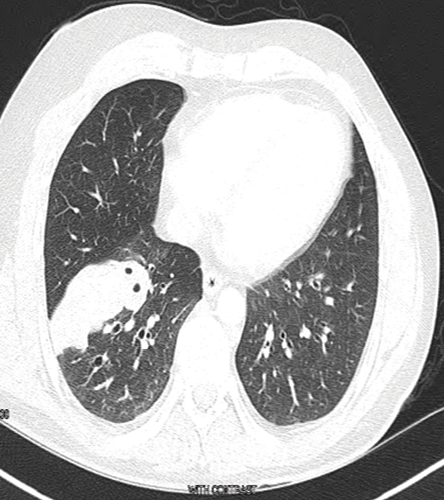
Figure 2: Computed tomography scan cross-section on admission.
The patient underwent CT guided biopsy and histopathology [Figures 3 and 4].
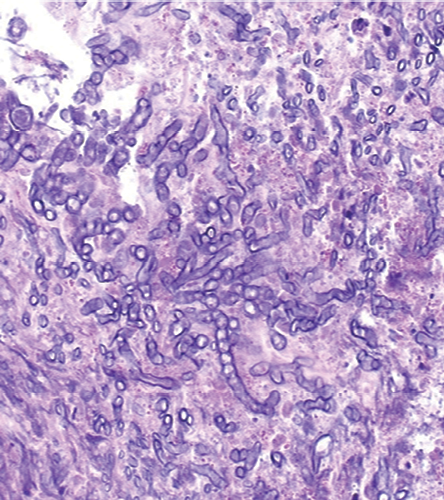
Figure 3: Hematoxylin and eosin stained lung biopsy, magnification=200×.

Figure 4: Gomori methenamine silver stained lung biopsy, magnification=200×.
Questions
- What is your diagnosis?
- How would you treat this disease?
Answer
Chest imaging showed a mass extending from the hilum to the right chest wall without cavitation or pleural effusion [Figure 2]. He underwent CT-guided lung biopsy, which showed fungal hyphae embedded within necrotic debris [Figure 3]. The hyphae were septate and acutely branching at 45 degrees consistent with aspergillus [Figure 4]. Upon review of the pulmonologist records, the patient was diagnosed and treated for allergic bronchopulmonary aspergillosis (ABPA) three years ago. The patient stopped seeing his pulmonologist ,but continued to treat himself with steroids for the ABPA. In light of this, the patient was diagnosed with invasive pulmonary aspergillosis (IPA) that complicated his ABPA. This was because he overly suppressed his immunity in the absence of professional follow-up. His steroids were temporarily stopped and he was started on voriconazole. On follow-up, at four and eight weeks, he had complete resolution of his pleuritic chest pain and shortness of breath.
Discussion
Pulmonary aspergillosis is a fungal lung disease with different faces. The host’s immune status and underlying structural lung disease play a pivotal role in determining the extent of pulmonary involvement.1 Presentation ranges from a primarily allergic disease to a life-threatening invasive illness. It is commonly caused by Aspergillus fumigatus. The incidence of this infection is increasing and it carries a potentially high morbidity and mortality.2
The fungus Aspergillus is acquired by inhalation of airborne spores, which are abundant in both indoor and outdoor environments.2 Rarely, the spores might be inoculated through the skin and subsequently disseminate to the lungs, brain, and other organs.2 Alveolar macrophages and circulating neutrophils prevent the conidia from invading the host tissues. In patients with impaired immunity, (i.e. prolonged neutropenia, hematological malignancy, advanced HIV infection, organ transplantation, and chronic granulomatous disease) and those on lengthy courses of high-dose corticosteroids and chemotherapy, the spores germinate into hyphae and a life-threatening fungal infection develops.1,2 Recently, Aspergillus is increasingly recognized as a potential pathogen in new, less immunocompromised patient populations such as critically ill patients and patients with chronic obstructive pulmonary disease (COPD).3,4
The development of Aspergillus-related pulmonary disease depends on the number and virulence of inoculated organisms as well as the immune status of the host. The clinical spectrum of the disease ranges from a saprophytic infection, like in aspergilloma, which develops in a pre-existing lung cavity caused by tuberculosis (TB), sarcoidosis, neoplasm or other disease processes, to an allergic disease, like in ABPA, or an invasive infection, like in IPA. Rarely, does one distinct Aspergillus-related entity may change to another, as in our case.2
IPA is the most severe form of this infection. Like other forms of pulmonary aspergillosis, A. fumigatous is the most commonly isolated species followed by A. flavus, A. niger and A. terrus.2 The fungal hyphae invade tissue and blood vessels causing thrombosis and tissue necrosis. Clinical features of IPA are nonspecific and include fever that does not respond to broad-spectrum antibiotics, dyspnea, dry cough, pleuritic chest pain, and hemoptysis.5 Early recognition of this lethal condition relies on a high index of suspicion that is based on patient’s risk factors and presentation, followed by appropriate imaging. Radiological studies, preferably chest CT, might show consolidation, nodules, cavitation, air crescent sign, halo sign, and reversed halo sign.5 However, a definite diagnosis hinges on obtaining appropriate bronchoscopic or percutaneous tissue samples for culture and histopathological identification.
The ability to detect Aspergillus antigens, namely galactomannan and beta-D-glucan in serum and bronchoalveolar lavage fluid (BAL), has improved our ability to diagnose this infection.6,7 Unfortunately, their sensitivity and specificity continue to be modest at best. Galactomannan can be positive in other invasive mycosis. It can be falsely positive in patients on pipracillin/tazobactam and falsely negative in patients taking voriconazole. Furthermore, detecting Aspergillus nuclear material by the polymerase chain reaction testing on serum, BAL, or tissue has been another development in the field and is currently undergoing testing.8
IPA is classified into one of three diagnosis categories: proven, probable, and possible IPA.9 Proven IPA can be established if there is a histopathological documentation of aspergillosis or a positive culture of Aspergillus from a normally sterile site and clinically or radiologically consistent features. If there is a mycological evidence of aspergillosis on microscopy or a positive culture for Aspergillus from sputum or BAL or a positive Aspergillus antigen assay in a susceptible host with consistent clinical features, then probable IPA is diagnosed. Possible IPA is documented if consistent clinical features of aspergillosis in a susceptible host are seen.
Voriconazole is the treatment of choice for patients with IPA.9 Amphotericin B or echinocandins are adjunctive treatments or alternative agents in patients who fail or cannot tolerate voriconazole.9 Patients with IPA are treated for several months, but the exact duration of therapy needs to be individualized based on the patient’s response.
Chronic necrotizing aspergillosis (CNA) is an indolent form of IPA.10 It develops in patients with underlying lung disease like COPD, previous pulmonary TB, or cystic fibrosis. It can also develop in patients with mild forms of immunosuppression, for example, patients on low dose steroids or those with chronic liver disease.10 Patients frequently complain of constitutional symptoms, chronic cough, and hemoptysis. The principles of diagnosis and management are identical to IPA.9
Tracheobronchial aspergillosis (TBA) is a rare manifestation of pulmonary aspergillosis.11 It has three forms: pseudomembranous, obstructive, and ulcerative. The former is characterized by extensive involvement of the lower airways, with sloughing of necrotic epithelium that in combination with endobronchial mucus forms pseudomembranes. In obstructive TBA, the inflammation and excessive mucus production obstructs the airways without invasion of the bronchial mucosa, and in ulcerative TBA, there are variable ulcerative or plaque-like lesions involving the bronchial walls. The above morphological forms may easily change and they could co-exist.11 Due to the lack of characteristic clinical or radiological features, TBA is a difficult diagnosis to make.11 Patients might have fever, dyspnea, cough, wheezing, or hemoptysis. In patients with isolated TBA, the chest X-ray and CT are commonly negative. Occasionally, atelectasis, bronchial wall thickening, or mucus plugging may be seen on CT. Bronchoscopy remains the most important diagnostic modality: it enables the direct visualization of endobronchial lesions and the collection of specimens for microbiological, pathological, and fungal marker testing.
Voriconazole is recommended as the initial therapy in the treatment of TBA.9 Lipid formulations of amphotericin B, systemic and/or nebulized, and echinocandins are considered alternative or adjunctive therapies. The prognosis of TBA is poor and the outcome depends on the immune status of the patient and how early the infection is diagnosed.
ABPA is a hypersensitivity lung disease associated with the inflammatory destruction of airways in response to Aspergillus species.12 ABPA develops in patients with a history of atopy, asthma, or cystic fibrosis. Patients with one of the aforementioned illnesses present with episodic wheezing, coughing and producing sputum containing brown plugs, pleuritic chest pain, and fever. Chest imaging at an early stage of the disease appears normal. During acute exacerbations, fleeting, mainly upper lobes, alveolar opacities might be noticed. As the disease progresses, central bronchiectasis and pulmonary fibrosis develop. High-resolution chest CT is very sensitive in demonstrating the above changes. Pulmonary function testing early on shows reversible obstructive pattern, which might become irreversible and associated with a restrictive pattern in later stages of the illness. ABPA diagnostic criteria includes: asthma, central bronchiectasis, A. fumigatus hyper-reactivity documented by elevated specific immunoglobulins or on skin testing, fleeting pulmonary opacities, and peripheral eosinophilia.2 Corticosteroids are the cornerstone of therapy and could prevent irreversible pulmonary damage. Itraconazole and voriconazole have a demonstrable steroid-sparing effect.9
Aspergilloma is a fungal ball that develops in an old lung cavity. Most patients with this entity are asymptomatic, although some might report hemoptysis that is variable in severity.13 The diagnosis of pulmonary aspergilloma relies on identifying consistent clinical and radiological features in addition to serological or mycological evidence of aspergillosis. Treatment of symptomatic patients entails systemic, endobronchial, or intracavitary antifungals.9 Surgical resection is reserved for cases with recurrent hemoptysis who fail antifungal therapy.
Disclosure
The authors declared no conflicts of interest.
References
- Segal BH, Walsh TJ. Current approaches to diagnosis and treatment of invasive aspergillosis. Am J Respir Crit Care Med 2006 Apr;173(7):707-717.
- Kousha M, Tadi R, Soubani AO. Pulmonary aspergillosis: a clinical review. Eur Respir Rev 2011 Sep;20(121):156-174.
- Samarakoon P, Soubani A. Invasive pulmonary aspergillosis in patients with COPD: a report of five cases and systematic review of the literature. Chron Respir Dis 2008;5(1):19-27.
- Khasawneh F, Mohamad T, Moughrabieh MK, Lai Z, Ager J, Soubani AO. Isolation of Aspergillus in critically ill patients: a potential marker of poor outcome. J Crit Care 2006 Dec;21(4):322-327.
- Ruhnke M, Böhme A, Buchheidt D, Donhuijsen K, Einsele H, Enzensberger R, et al; Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Oncology (DGHO). Diagnosis of invasive fungal infections in hematology and oncology--guidelines of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Oncology (DGHO). Ann Hematol 2003 Oct;82(Suppl 2):S141-S148.
- Pfeiffer CD, Fine JP, Safdar N. Diagnosis of invasive aspergillosis using a galactomannan assay: a meta-analysis. Clin Infect Dis 2006 May;42(10):1417-1427.
- De Vlieger G, Lagrou K, Maertens J, Verbeken E, Meersseman W, Van Wijngaerden E. Beta-D-glucan detection as a diagnostic test for invasive aspergillosis in immunocompromised critically ill patients with symptoms of respiratory infection: an autopsy-based study. J Clin Microbiol 2011 Nov;49(11):3783-3787.
- Hizel K, Kokturk N, Kalkanci A, Ozturk C, Kustimur S, Tufan M. Polymerase chain reaction in the diagnosis of invasive aspergillosis. Mycoses 2004 Aug;47(7):338-342.
- Walsh TJ, Anaissie EJ, Denning DW, Herbrecht R, Kontoyiannis DP, Marr KA, et al; Infectious Diseases Society of America. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis 2008 Feb;46(3):327-360.
- Gefter WB, Weingrad TR, Epstein DM, Ochs RH, Miller WT. “Semi-invasive” pulmonary aspergillosis: a new look at the spectrum of aspergillus infections of the lung. Radiology 1981 Aug;140(2):313-321.
- Wu N, Huang Y, Li Q, Bai C, Huang HD, Yao XP. Isolated invasive Aspergillus tracheobronchitis: a clinical study of 19 cases. Clin Microbiol Infect 2010 Jun;16(6):689-695.
- Agarwal R. Allergic bronchopulmonary aspergillosis. Chest 2009 Mar;135(3):805-826.
- Kawamura S, Maesaki S, Tomono K, Tashiro T, Kohno S. Clinical evaluation of 61 patients with pulmonary aspergilloma. Intern Med 2000 Mar;39(3):209-212.