During pregnancy two-thirds of women experience alteration in their sleep. These sleep alterations, which begin in the first trimester, appear to be influenced by the pronounced alterations in the levels of reproductive hormones that occur during gestation.1 Also, rotating shifts and night shifts in the workplace predispose pregnant women to decreased sleep quality and duration, which may adversely affect the pregnancy outcome.2
Sleep deprivation during pregnancy affects both maternal and fetal well-being. In the mother, it is associated with prolonged labor, preeclampsia, glucose intolerance, postpartum depression and preterm labor, and the infants can be small for their gestational age.1,3-5
The mechanism behind these complications are poorly understood. Previous studies have observed higher levels of inflammatory cytokines in sleep deprived pregnant mothers. Inflammation can cause preterm labor through its action on prostaglandin biosynthesis. Similarly, postpartum depression and sleep disturbances are explained by inflammation as an underlying mechanism.1
The inherent property of immune activation (inflammation) is generation of reactive oxygen species, which can lead to depletion of antioxidants, and genesis of oxidative stress.6 Oxidative stress, in turn, can enhance inflammation through its property of inducing proinflammatory cytokines. Hence, oxidative stress and inflammation are inseparably connected.7 Since inflammation is strongly believed to play a role in the complications of sleep deprivation, it is worthwhile to study the status of inflammation and oxidants in sleep-deprived pregnant mothers.
Homocysteine is a sulphur containing amino acid derived from the demethylation of methionine.8 Factors like stress, some vitamin B deficiencies, and genetic defects in the enzymes involved in homocysteine metabolism can elevate homocysteine levels.9 During pregnancy, high homocysteine levels have been associated with risks of preeclampsia and premature delivery, and with neural tube defect, and low birth weight in infants.10
For the first time, we aimed to study whether maternal sleep deprivation occurring during the second and third trimester of pregnancy could alter fetal well-being, including birth weight and APGAR score, by altering inflammatory status, oxidative stress, and homocysteine levels.
Methods
This study was conducted in the Department of Biochemistry, Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry, India, in collaboration with the Department of Obstetrics and Gynaecology and was approved by the Institute Research Council and Institute Ethics Committee. Sleep deprivation was assessed using the Pittsburgh Sleep Quality Index (PSQI) score, which assesses sleep quality over a month. It consists of 19 self-rated questions and five questions rated by the subjects bed partner or room-mate. The latter questions are not included in the scoring. The 19 self-rated questions assess sleep quality, latency, duration, and the frequency and severity of sleep related problems. These questions are divided into seven components, each weighted equally on a score of zero to three. Total score ranged from zero to 21 and the higher the score, the worse the sleep. The study included two groups of pregnant women in their second or third trimester, the cases who were sleep deprived (n=30) and the control group (n=38) who had adequate sleep. Pregnant women who had complications in pregnancy and neuropsychiatric disorders were excluded from the study. After taking a written informed consent, 4ml of venous blood samples were collected from the study subjects at the time of admission before the onset of labor. Fetal birth weight and APGAR score were noted at the time of delivery.
The levels of protein-bound sialic acid (PBSA), high-sensitivity C-reactive protein (hsCRP), malondialdehyde (MDA), protein carbonyl (PCO), vitamin B12, and folic acid in maternal serum were measured. Homocysteine levels were estimated in plasma. The PBSA of serum was measured by Aminoff’s method based on the principle of colorimetry. Serum hsCRP levels were estimated by ELISA using a commercial kit (Diagnostic Biochem Canada Inc., Canada). Serum MDA were estimated by thiobarbituric acid by Sotah’s method using spectrophotometry. Serum PCO was measured using Levine’s method modified by Chakroborthy using spectrophotometry. Homocysteine, folate, and vitamin B12 levels were assayed in chemiluminescence (ADVIA Centaur, Siemens, Japan) by direct competitive immunoassay.
Results were expressed as the mean±standard deviation (SD). The comparison between the case and the control group was done using the Student’s t-test and Mann-Whitney U tests for parametric and nonparametric data, respectively. Correlation analysis was done using Pearson’s correlation coefficient. A p-value <0.050 was considered significant.
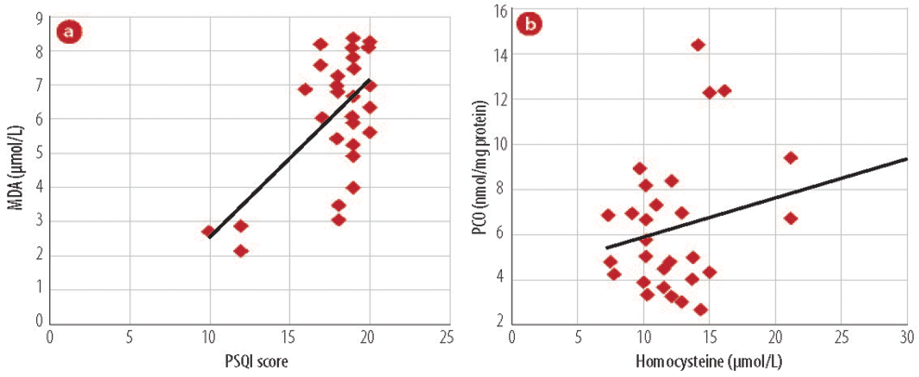
Figure 1: Association of oxidant markers to (a) PSQI score and malondialdehyde (MDA). (b) Homocysteine with protein carbonyl (PCO) in study groups.
Results
The study groups were matched for gestational age. There was no significant difference in the age, weight, and hemoglobin between the case and control groups [Table 1]. Sleep deprived pregnant women had high levels of hsCRP and PBSA, which was statistically significant. Lipid peroxidation and protein carbonyls were significantly higher in the case group [Table 2]. Homocysteine levels were significantly higher in sleep-deprived mothers. There was no difference in folate and vitamin B12 levels [Table 3].
Table 1: Characteristics of the studied population.
|
Age (years) |
26.5±2.1 |
27.5±4.0 |
0.925 |
|
Weight (kg) |
56.1±3.8 |
55±8 |
0.757 |
|
Gestational age (weeks) |
35.2±4.1 |
34.1 ±3.4 |
0.757 |
|
Hemoglobin |
9.6±1.5 |
10.4±2.4 |
0.802 |
PSQI: Pittsburgh Sleep Quality Index.
Birth weight and APGAR score taken at the time of delivery were not found to be different between study subjects [Table 4]. Cases were further subgrouped depending on the mean PSQI score [Table 5]. Group I included patients with a PSQI score of less than 18 and group II with a score greater than 18. Markers of lipids and protein oxidation injury were significantly higher in group II than group I. Fetal birth weight and APGAR score were found to be lower in group II than group I.
Among the cases, lipid peroxidation exhibited a positive association with PSQI score. The levels of homocysteine correlated directly with PCO levels. PSQI score was plotted against MDA (r=0.610; p<0.001) and PCO was plotted against homocysteine levels (r=0.380; p=0.038) [Figure 1].
Table 2: Inflammatory and oxidative stress marker levels in sleep-deprived pregnant women (case group) and those who had adequate sleep (control group).
|
hsCRP (ng/mL) |
3697.5±3508.8 |
5520.8±3708.3 |
0.042 |
|
MDA (µmol/L) |
4.1±1.2 |
6.1± 1.8 |
0.001 |
|
Protein carbonyls (nmol/mg of protein) |
3.1±1.1 |
6.5±3.1 |
0.000 |
*p<0.050 when compared with pregnant women with adequate sleep using Student’s t-test and Mann-Whitney U test for parametric and nonparametric data, respectively; hsCRP: high-sensitivity C-reactive protein; MDA: malondialdehyde; PBSA: protien bound sialic acid.
Table 3: Serum levels of homocysteine and its vitamin determinants in pregnant women who were sleep deprived (case group) and who had adequate sleep (control group).
|
Folate
(ng/mL) |
8.2±6.3 |
7.7±4.1 |
0.063 |
|
Vitamin B12 (pg/mL) |
220.8±85.4 |
219.5±65.4 |
0.947 |
*p<0.050 when compared with pregnant women with adequate sleep using Student’s t-test and Mann-Whitney U test for parametric and nonparametric data, respectively.
Discussion
We measured subjective sleep quality in late pregnancy using the PSQI, which has been validated and found to be useful in pregnancy research.11,12 In the latter part of pregnancy women experience more sleep disturbances and potential risk factors for depressive symptoms.11 The subjective perception of sleep rather than the objective measurement is thought to predisposes women to complications associated with sleep deprivation.12
Table 4: Markers of fetal outcome in pregnant women who were sleep deprived (case group) and who had adequate sleep (control group).
|
Preterm delivery |
7.8% (n=3) |
6.6% (n=2) |
0.916 |
|
Birth weight (kg) |
2.8±0.4 |
2.7±0.4 |
0.603 |
*p<0.050 when compared with pregnant women with adequate sleep using Student’s t-test and chi-square test for preterm delivery.
Sleep loss leads to nonspecific activation of leukocytes and a state of low grade inflammation.13 In our study, we observed hsCRP and PBSA to be significantly increased in sleep-deprived pregnant women when compared to pregnant women with adequate sleep. Our results are in agreement with previous study that stated that poor sleep quality and continuity were associated with higher CRP levels during pregnancy.14 Sleep loss induces an increase in inflammatory mediators through its action on activating transcription factor NF-ĸ.15 Sleep loss upregulates several proinflammatory cytokines, which in turn increases CRP levels. A study conducted by Meier-Ewert et al,16 showed elevated hsCRP, a stable marker of inflammation in both acute total and short-term sleep deprivation.
Table 5: Oxidative stress marker levels and fetal outcomes in the subgroups of pregnant women with sleep deprivation using PSQI score (group I score <18 and group II score >18).
|
Folate (ng/mL) |
4.4±3.8 |
3.8±4.3 |
0.476 |
|
Vitamin B12 (pg/mL) |
222.6±57.0 |
217.0±74.0 |
0.712 |
|
Homocysteine (µmol/L) |
13.4±4.3 |
14.3±9.1 |
0.081 |
|
hsCRP (ng/mL) |
6108.3±3877.0 |
5006.0±3599.0 |
0.331 |
|
Malendialdehyde (µmol/L) |
5.2±1.8 |
6.8±1.4* |
0.001 |
|
Protein carbonyls
(nmol/mg protein) |
6.5±1.1 |
7.8±3.3* |
0.034 |
|
PBSA (µg/mg protein) |
3.7±0.71 |
3.8±0.8 |
0.131 |
|
Birth weight (kg) |
2.9±0.4 |
2.6±0.4* |
0.021 |
*p<0.050 when compared with pregnant women with low sleep deprivation using Student’s t-test and Mann-Whitney U test for parametric and nonparametric data, respectively. hsCRP: high-sensitivity C-reactive protein; PBSA: protein-bound sialic acid.
It has been hypothesized that the brain faces an oxidative challenge when it is in a wakeful state and sleep may allow the removal of free radicals.17 Hence, sleep deprivation may cause oxidative stress. The consequences of sleep deprivation seem to be mediated by biochemical factors since they are reversible with sleep. We found a significant increase in MDA in sleep-deprived pregnant women when compared to pregnant women who had adequate sleep. Since MDA is one of the major aldehydes formed after the breakdown of lipid hydroperoxides, it is considered as a good biomarker of the involvement of free radical damage in pathologies associated with oxidative stress.
Sleep apnea, a disorder with large sleep interruption, has been a focus of studies of oxidative stress in sleep disruption. One study reported increased oxidative stress in patients with sleep apnea.18 Monocyte expression of heat shock proteins, tumor necrosis factor alpha, and MDA were upregulated in subjects with sleep apnea indicating the clear evidence of sleep disturbances in induction of pro-oxidant state.19 Insomnia in postmenopausal women has been associated with increased lipid peroxidation levels.20 Additionally, in sleep deprived animal models, large variations in the levels of antioxidant defense mechanisms were reported in the brain and peripheral tissues, which had an impact on the animals psychological reactions.21-23
Sialic acid comprises of a family of acetylated derivatives of neuraminic acid, which act as acute phase proteins and elevated in numerous inflammatory conditions like diabetes, cancer, and renal diseases.24-26 We found significantly raised levels of PBSA in sleep deprived patients compared to those with adequate sleep. We also found a positive relationship between lipid peroxidation and sleep quality. Previous studies have found significant improvements in quality of sleep with supplementation of natural antioxidants.27,28 The Therapeutic administration of melatonin in sleep deprivation has been explored.29,30 One of the mechanisms by which it improves sleep is by its partial inhibitory action on the expression of NF-ĸ thereby alleviating the chronic inflammation and oxidative stress.31,32
Direct damage to proteins or chemical modification of amino acids in proteins during oxidative stress and glycoxidation can give rise to protein carbonyls, which may serve as biomarkers for general oxidative stress. Using the presence of carbonyl groups as the evidence of protein oxidation, it was established that protein oxidation was associated with oxidative stress and a number of diseases.33 To our knowledge, we are the first to estimate the level of carbonylation of plasma proteins in sleep-deprived pregnant women and we found them to be significantly high. Ramanathan and Siegel34 studied the interaction between sleep deprivation and hypoxia in a rat model and measured the levels of protein carbonyls as marker of protein oxidation. They concluded that in the presence of hypoxia short-term insomnia may be an adaptive reaction to prevent oxidative stress.
A study by Martins et al,35 reported hyperhomocystenemia in bus drivers working on a shift basis. The severity of sleep disruption was strongly associated with homocysteine levels in ischemic stroke patients with obstructive sleep apnea.36 Our study showed significantly higher homocysteine concentrations in sleep-deprived pregnant women compared to the control group in spite of normal folate and B12 status. Although elevated plasma total homocysteine concentration is a sensitive marker of folate/vitamin B12 status, our study suggests that it does not hold true in the case of pregnant women. This may be because pregnant women are supplemented with folate and B12 throughout pregnancy. Hyperhomocysteinemia in cases without signs of folate or vitamin B12 deficiency may be the consequence of stress induced by sleep disturbances during pregnancy. However, levels of methylmalonic acid, the sensitive marker of vitamin B12 and other homocysteine vitamin determinants, were not measured in this study.
Our study showed a significant positive correlation of homocysteine with PCO. Homocysteine is oxidised readily and during the process it promotes oxidative stress via reactive oxygen species (ROS) generation.37 The additional carbon in the homocysteine side chain permits it to exist as a thiolactone, which reacts with the Lys residues to form isopeptide bonds. Homocystamides render proteins more prone to oxidation forming PCO.38 In the study conducted in elderly patients with obstructive sleep apnea syndrome, the degree of oxidative stress associated positively with the homocysteine levels.39
We hypothesised that the increased inflammation, oxidative stress, and homocysteine levels in sleep-deprived pregnant women would have a negative impact on the fetal outcome. However, the birth weight and APGAR scores of the newborns of sleep-deprived mothers showed no significant difference when compared to the newborns of mothers with adequate sleep. Comparison of subgroups of the cases with sleep deprivation showed high oxidation markers and low APGAR and birth weight in mothers with high sleep deprivation than in mothers with low sleep deprivation. It has been speculated that sleep-disordered breathing during pregnancy can induce intermittent hypoxia, which may potentiate placental ischemia, precipitating oxidative stress, and endothelial activation. A short period of maternal hypoxia can decrease the fetal heart rate and breathing.40 In this framework, previous data suggest that maternal sleep deprivation is one of the independent risk factors for intrauterine growth restriction and small for gestational age infants.41,42 Franklin and colleagues43 reported higher incidence of small for gestational age infants in snorers. However, studies by Loube et al,44 and Hedman et al,45 did not find a significant difference in birth weight and APGAR scores between snorers and nonsnorers. Another complication of sleep deprivation is preterm labor.46 We did not find any difference in the incidence of preterm labor among cases, which could be due to the small sample size. A larger sample size may have helped in stratifying groups with sleep disturbances. Polysomnagraphic recording of study subjects would also have thrown a better light on the objective details of sleep. The lack of follow-up after delivery to check the status of the sleep pattern in the study group and the well-being of newborn was also a limitation of this study.
Conclusion
Since sleep disturbances during pregnancy showed a significant increase in inflammatory status, oxidative stress, and homocysteine levels, and a negative impact on fetal well-being, sleep relaxing exercises could be considered by women during pregnancy.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
references
- Chang JJ, Pien GW, Duntley SP, Macones GA. Sleep deprivation during pregnancy and maternal and fetal outcomes: is there a relationship? Sleep Med Rev 2010 Apr;14(2):107-114.
- Bonzini M, Palmer KT, Coggon D, Carugno M, Cromi A, Ferrario MM. Shift work and pregnancy outcomes: a systematic review with meta-analysis of currently available epidemiological studies. BJOG 2011 Nov;118(12):1429-1437.
- Abeysena C, Jayawardana P. Sleep deprivation, physical activity and low income are risk factors for inadequate weight gain during pregnancy: a cohort study. J Obstet Gynaecol Res 2011 Jul;37(7):734-740.
- Micheli K, Komninos I, Bagkeris E, Roumeliotaki T, Koutis A, Kogevinas M, et al. Sleep patterns in late pregnancy and risk of preterm birth and fetal growth restriction. Epidemiology 2011 Sep;22(5):738-744.
- Williams MA, Miller RS, Qiu C, Cripe SM, Gelaye B, Enquobahrie D. Associations of early pregnancy sleep duration with trimester-specific blood pressures and hypertensive disorders in pregnancy. Sleep 2010 Oct;33(10):1363-1371.
- Yoon JW, Pahl MV, Vaziri ND. Spontaneous leukocyte activation and oxygen-free radical generation in end-stage renal disease. Kidney Int 2007 Jan;71(2):167-172.
- Kim CH, Vaziri ND. Hypertension promotes integrin expression and reactive oxygen species generation by circulating leukocytes. Kidney Int 2005 Apr;67(4):1462-1470.
- Finkelstein JD. Methionine metabolism in mammals. J Nutr Biochem 1990 May;1(5):228-237.
- Loscalzo J. The oxidant stress of hyperhomocyst(e)inemia. J Clin Invest 1996 Jul;98(1):5-7.
- Hague WM. Homocysteine and pregnancy. Best Pract Res Clin Obstet Gynaecol 2003 Jun;17(3):459-469.
- Skouteris H, Wertheim EH, Germano C, Paxton SJ, Milgrom J. Assessing sleep during pregnancy: a study across two time points examining the Pittsburgh Sleep Quality Index and associations with depressive symptoms. Womens Health Issues 2009 Jan-Feb;19(1):45-51.
- Bei B, Milgrom J, Ericksen J, Trinder J. Subjective perception of sleep, but not its objective quality, is associated with immediate postpartum mood disturbances in healthy women. Sleep 2010 Apr;33(4):531-538.
- Faraut B, Boudjeltia KZ, Vanhamme L, Kerkhofs M. Immune, inflammatory and cardiovascular consequences of sleep restriction and recovery. Sleep Med Rev 2012 Apr;16(2):137-149.
- Sluzewska A, Rybakowski J, Bosmans E, Sobieska M, Berghmans R, Maes M, et al. Indicators of immune activation in major depression. Psychiatry Res 1996 Oct;64(3):161-167.
- Krueger JM, Obál FJ, Fang J, Kubota T, Taishi P. The role of cytokines in physiological sleep regulation. Ann N Y Acad Sci 2001 Mar;933:211-221.
- Meier-Ewert HK, Ridker PM, Rifai N, Regan MM, Price NJ, Dinges DF, et al. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol 2004 Feb;43(4):678-683.
- Gopalakrishnan A, Ji LL, Cirelli C. Sleep deprivation and cellular responses to oxidative stress. Sleep 2004 Feb;27(1):27-35.
- Carpagnano GE, Kharitonov SA, Resta O, Foschino-Barbaro MP, Gramiccioni E, Barnes PJ. 8-Isoprostane, a marker of oxidative stress, is increased in exhaled breath condensate of patients with obstructive sleep apnea after night and is reduced by continuous positive airway pressure therapy. Chest 2003 Oct;124(4):1386-1392.
- Lavie L, Dyugovskaya L, Golan-Shany O, Lavie P. Heat-shock protein 70: expression in monocytes of patients with sleep apnoea and association with oxidative stress and tumour necrosis factor-alpha. J Sleep Res 2010 Mar;19(1 Pt 2):139-147.
- Hachul de Campos H, Brandão LC, D’Almeida V, Grego BH, Bittencourt LR, Tufik S, et al. Sleep disturbances, oxidative stress and cardiovascular risk parameters in postmenopausal women complaining of insomnia. Climacteric 2006 Aug;9(4):312-319.
- D’Almeida V, Lobo LL, Hipólide DC, de Oliveira AC, Nobrega JN, Tufik S. Sleep deprivation induces brain region-specific decreases in glutathione levels. Neuroreport 1998 Aug;9(12):2853-2856.
- Everson CA, Laatsch CD, Hogg N. Antioxidant defense responses to sleep loss and sleep recovery. Am J Physiol Regul Integr Comp Physiol 2005 Feb;288(2):R374-R383.
- Vollert C, Zagaar M, Hovatta I, Taneja M, Vu A, Dao A, et al. Exercise prevents sleep deprivation-associated anxiety-like behavior in rats: potential role of oxidative stress mechanisms. Behav Brain Res 2011 Oct;224(2):233-240.
- Kökoğlu E, Sönmez H, Uslu E, Uslu I. Sialic acid levels in various types of cancer. Cancer Biochem Biophys 1992 Oct;13(1):57-64.
- Crook M. Type 2 diabetes mellitus: a disease of the innate immune system? An update. Diabet Med 2004 Mar;21(3):203-207.
- Ozben T. Elevated serum and urine sialic acid levels in renal diseases. Ann Clin Biochem 1991 Jan;28(Pt 1):44-48.
- Sun J. Morning/evening menopausal formula relieves menopausal symptoms: a pilot study. J Altern Complement Med 2003 Jun;9(3):403-409.
- Howatson G, Bell PG, Tallent J, Middleton B, McHugh MP, Ellis J. Effect of tart cherry juice (Prunus cerasus) on melatonin levels and enhanced sleep quality. Eur J Nutr 2011.
- Campos FL, da Silva-Júnior FP, de Bruin VM, de Bruin PF. Melatonin improves sleep in asthma: a randomized, double-blind, placebo-controlled study. Am J Respir Crit Care Med 2004 Nov;170(9):947-951.
- Rogers NL, Dinges DF, Kennaway DJ, Dawson D. Potential action of melatonin in insomnia. Sleep 2003 Dec;26(8):1058-1059.
- Wang YT, Chen SL, Xu SY. [Effect of melatonin on the expression of nuclear factor-kappa B and airway inflammation in asthmatic rats]. Zhonghua Er Ke Za Zhi 2004 Feb;42(2):94-97.
- El-Helaly M, Abu-Hashem E. Oxidative stress, melatonin level, and sleep insufficiency among electronic equipment repairers. Indian J Occup Environ Med 2010 Sep;14(3):66-70.
- Berlett BS, Stadtman ER. Protein oxidation in aging, disease, and oxidative stress. J Biol Chem 1997 Aug;272(33):20313-20316.
- Ramanathan L, Siegel JM. Sleep deprivation under sustained hypoxia protects against oxidative stress. Free Radic Biol Med 2011 Nov;51(10):1842-1848.
- Martins PJ, D’Almeida V, Vergani N, Perez AB, Tufik S. Increased plasma homocysteine levels in shift working bus drivers. Occup Environ Med 2003 Sep;60(9):662-666.
- Chen M, Wu B, Ye X, Zhou Z, Yue X, Wang Q, et al. Association between plasma homocysteine levels and obstructive sleep apnoea in patients with ischaemic stroke. J Clin Neurosci 2011 Nov;18(11):1454-1457.
- Loscalzo J. The oxidant stress of hyperhomocyst(e)inemia. J Clin Invest 1996 Jul;98(1):5-7.
- Sibrian-Vazquez M, Escobedo JO, Lim S, Samoei GK, Strongin RM. Homocystamides promote free-radical and oxidative damage to proteins. Proc Natl Acad Sci U S A 2010 Jan;107(2):551-554.
- Wang L, Li J, Xie Y, Zhang XG. Association between serum homocysteine and oxidative stress in elderly patients with obstructive sleep apnea/hypopnea syndrome. Biomed Environ Sci 2010 Feb;23(1):42-47.
- Pien GW, Schwab RJ. Sleep disorders during pregnancy. Sleep 2004 Nov;27(7):1405-1417.
- Abeysena C, Jayawardana P, DE A Seneviratne R. Maternal sleep deprivation is a risk factor for small for gestational age: a cohort study. Aust N Z J Obstet Gynaecol 2009 Aug;49(4):382-387.
- Abeysena C, Jayawardana P. Sleep deprivation, physical activity and low income are risk factors for inadequate weight gain during pregnancy: a cohort study. J Obstet Gynaecol Res 2011 Jul;37(7):734-740.
- Franklin KA, Holmgren PA, Jönsson F, Poromaa N, Stenlund H, Svanborg E. Snoring, pregnancy-induced hypertension, and growth retardation of the fetus. Chest 2000 Jan;117(1):137-141.
- Hedman C, Pohjasvaara T, Tolonen U, Suhonen-Malm AS, Myllylä VV. Effects of pregnancy on mothers’ sleep. Sleep Med 2002 Jan;3(1):37-42.
- Loube DI, Poceta JS, Morales MC, Peacock MD, Mitler MM. Self-reported snoring in pregnancy. Association with fetal outcome. Chest 1996 Apr;109(4):885-889.
- Okun ML, Schetter CD, Glynn LM. Poor sleep quality is associated with preterm birth. Sleep 2011 Nov;34(11):1493-1498.