Spinal subarachnoid block is still the first choice anesthesia in lower abdominal and lower limb surgeries because it blunts the “stress response” to surgery and decreases intraoperative blood loss and the incidence of postoperative thromboembolic events.1 It is a cost-effective option and also produces a rapid onset, superior blockade with a lower failure rate, but has the drawbacks of shorter duration of block and lack of postoperative analgesia. In recent years, use of intrathecal adjuvants has gained popularity with the aim of prolonging the duration of the block and achieving better success rates and faster recovery.2 Gynecological surgeries are often associated with severe pain requiring a well-planned analgesia regimen to ensure adequate patient-comfort, early mobilization, and to decrease stay time in the hospital/post-anesthesia care unit (PACU) enabling patients to return to their normal activities quicker.
The use of different techniques and drugs in order to prolong the duration of regional anesthesia and achieve post-hysterectomy pain relief have been studied.3 Opioids are commonly administered as an adjuvant, but urinary retention, respiratory depression, pruritus, and, occasionally, severe nausea and vomiting may limit their use.4-7
Other classes of drugs such as epinephrine,8 neostigmine,9 magnesium,10 midazolam,11 ketamine,12 and clonidine13 have been added to intrathecal local anesthetics in an attempt to prolong analgesia and reduce the incidence of adverse events.
Dexmedetomidine, a highly selective alpha-2 adrenoceptor agonist with sedative and analgesic properties has been found to reduce the anesthetic requirements during general anesthesia.14 It has been found to exert its analgesic actions both at the spinal and supraspinal levels.15
A significant prolongation in the duration of sensory and motor block with dexmedetomidine used as an intrathecal additive for 0.5% heavy bupivacaine has been demonstrated previously.16 Previous studies have described the intrathecal use of dexmedetomidine in a wide range (2–10μg).17-20
To compare the subarachnoid block characteristics (onset and duration of sensory and motor block) 5µg and 10µg dexmedetomidine was added to 15mg 0.5% hyperbaric bupivacaine given intrathecally for spinal anesthesia. Our study also sought to assess the need for postoperative analgesia and if there was any increase in dexmedetomidine-dose dependent side effects.
METHODS
A total of 100 adult patients were randomly allocated to one of two treatment groups (n=50 in each group) using a computer-generated random number list. Female patients with American Society of Anesthesiologists (ASA) physical status classification I & II, aged 35–60 years assigned to undergo elective abdominal hysterectomy under spinal anesthesia were included in the study. Patients were excluded if they refused to take part in the study, had any known allergy or contraindication to bupivacaine or dexmedetomidine, or any hepatic, renal, or cardiopulmonary abnormality, tubo-ovarian mass with ascites, alcoholism, diabetes, spinal cord deformities, bleeding diathesis, local skin site infections, or were on long-term analgesic or anticoagulant therapy.
Depending on their assigned group, patients received 5μg (D5 group) or 10μg (D10 group)dexmedetomidine along with 15mg (3ml) of 0.5% hyperbaric bupivacaine in the subarachnoid space.
Permission to perform the study was given by the institutional ethics committee, and written informed consent was taken from each patient.
In preoperative assessment, patients were asked about any history of drug allergy, previous operations, or prolonged drug treatment. General and systemic examinations and assessment of the airway were performed and patients were asked to fast for a minimum of six hours before the operation. All patients received diazepam 10mg orally the night before surgery as per preanesthetic check-up direction. Patients also received ranitidine 150mg orally the night before surgery and the following morning.
All patients were clinically examined in the preoperative period, when the whole procedure was explained. All patients underwent tests to determine the following: hemoglobin concentration, total and differential leukocyte count, erythrocyte sedimentation rate, platelet count, blood sugar, blood urea, creatinine, and liver function. A 12 lead electrocardiogram (ECG) and chest X-ray were also taken. On entering the patient in the operative room, standard intraoperative monitors were attached (ECG, pulse oximeter, non-invasive blood pressure) and baseline parameters were recorded using a Philips IntelliVue MP20 monitor. The patients were preloaded with Lactated Ringer’s solution 10ml/kg.
The anesthetic technique was standardized for all patients. Patients were in a seated position for lumbar puncture at the L3-L4 intervertebral space in median approach with a 26 Gauge spinal (Quincke) needle using aseptic precautions.
The D5 group received intrathecal injection of 0.5% hyperbaric bupivacaine 15mg (3ml) with dexmedetomidine 5μg injection (0.5ml Dextomid 100μg/ml; Neon Laboratories Ltd, Mumbai, India) which was diluted with normal saline to 5ml (10μg/ml) and 0.5ml (5μg) of this solution was added to 3ml bupivacaine with a 1ml syringe. The D10 group received intrathecal 0.5% hyperbaric bupivacaine 15mg (3ml) with dexmedetomidine 10μg injection (0.5ml of dexmedetomidine was diluted with normal saline to 2.5ml (20μg/ml) and 0.5ml (5μg) of this solution was added to 3ml bupivacaine with a 1ml syringe). For blinding purposes, the final volume of injected drug was kept constant at 3.5ml for both the groups and the solutions were prepared by resident doctors not taking part in the study. Intrathecal injection was given over approximately 10 seconds. Patients were made to lie supine immediately after the injection.
Oxygen (2L/min) was administered via a mask if the pulse oximeter reading decreased below 90%. Any decrease in systolic blood pressure (SBP <100mmHg) or a drop greater than 20% from baseline value was considered as hypotension and SBP<90mmHg was treated with slow intravenous (iv) mephentermine 6mg, which was repeated after five minutes if SBP was not corrected. Tachycardia was defined as a heart rate (HR) greater than 100 beats per minute (bpm) and bradycardia when HR was less than 60bpm. When HR fell below 50bpm atropine 0.5mg iv injection was administered. The incidence of adverse effects, such as nausea, vomiting, shivering, pruritus, respiratory depression, sedation, bradycardia, and hypotension were recorded. Sensory testing was assessed by loss of pinprick sensation to 23G hypodermic needle and dermatomes levels were tested every two minutes until the highest level had stabilized on consecutive tests. On achieving T8 sensory blockade level, surgery was allowed. Testing was then conducted every 10 minutes until the point of two-segment regression of the block was observed. Further testing was performed at 20-minute intervals until the recovery of S2 dermatome. The gynecologist, patient, and the observing anesthesiologist were blinded to the patient group. Data regarding the highest dermatome level of sensory blockade, the time to reach this level from the time of injection, time to S1 level sensory regression, time to urination, and incidence of side effects were recorded.
Postoperatively, the pain score was recorded by using visual analogue scale (VAS) which measured pain on a scale of 0 to 10. A score was taken every hour for the first six hours then every two hours for the next eight hours, and then every four hours until 24 hours post-surgery. Diclofenac sodium (75 mg) was given intramuscularly as rescue analgesia when the VAS score was less than four.
The sample size was based on a crossover pilot study of 12 patients and was selected to detect a projected difference of 10% between the groups for two-segment regression time of sensory blockade for a type 1 error of 0.05 and a power of 0.80. The average sensory regression time in each group was 150 minutes and, based on previous studies, within-group standard deviation was 25 minutes. We calculated that we needed at least 45 patients per group to be able to reject the null hypothesis.
Raw data were entered into a Microsoft Excel spreadsheet and analyzed using standard statistical software SPSS statistical package (SPSS Inc., Chicago, USA) version 18.0. Categorical variables were analyzed using the Pearson’s chi-square test. Normally distributed continuous variables were analyzed using the independent sample t-test and a p-value less than 0.050 was considered statistically significant.
Table 1: Comparison of demographic data between the two study groups (n=50 per group).
|
Age (years) |
45.2±15.3 |
44.7±14.1 |
0.879 |
|
Bodyweight (kg) |
61.2±5.1 |
60.67±5.4 |
0.719 |
|
ASA physical status (I/II) |
42/8 |
38/12 |
0.810 |
|
Height (cm) |
151.6±7.4 |
152.1±8.1 |
0.371 |
Table 2: Indications for hysterectomies in the two patient groups.
|
Menorrhagia (including abnormal bleeding) |
18(36) |
20(40) |
|
Early cervical intraepithelial neoplasia |
5(10) |
4(8) |
|
Endometriosis |
7(14) |
6(12) |
|
Dysmenorrhea |
7(14) |
5(10) |
|
Chronic pelvic pain |
5(10) |
6(12) |
*n(%).
Results
We recruited a total of 100 subjects (50 per group) and there were no dropouts or failed spinal cases. The age, body weight, sex distribution, ASA status, height, and duration of surgery in the two groups were comparable [Table 1]. Indications for elective hysterectomies were similar in the two groups and had no statistical significance [Table 2]. Table 3 shows the preoperative HR, systolic, diastolic and mean BP, and hemoglobin levels of the two groups, which were also comparable. Onset of both sensory and motor block was earlier in the D10 than the D5 group but they were not statistically significant (p>0.050). Sensory and motor block durations were greater in the D10 group than the D5 group and the results were statistically significant
(p<0.050) [Table 4]. The mean time from subarachnoid block to first request for pain medication (i.e. the duration of analgesia) was 366.6 min in the D10 group but 242.2 min in the D5 group (p<0.050) [Table 4]. The D10 group required less diclofenac sodium injection (121.3mg vs 221.1mg) as rescue analgesics than patients in the D5 group in the 24 hour postoperative period and this difference was statistically significant (p<0.050).
Figure 1 and 2 shows the HR and mean arterial pressure among the two groups, respectively. VAS scores between the two treatment groups was higher in the D5 group than the D10 group [Figure 3]. The D10 group suffered more from bradycardia than the D5 group (p<0.050). The side effects experienced by each group were comparable [Table 5].
Table 3: Comparison of preoperative vitals between the two study groups.
|
Hemoglobin (gm/dl) |
10.0±1.3 |
11.0±1.4 |
0.790 |
|
Systolic BP (mm Hg) |
121.0±13.8 |
128.4±14.9 |
0.111 |
|
Diastolic BP (mm Hg) |
81.5±12.2 |
87.0±12.9 |
0.194 |
|
Mean arterial pressure |
92.9±12.4 |
99.0±13.2 |
0.161 |
*mean±SD
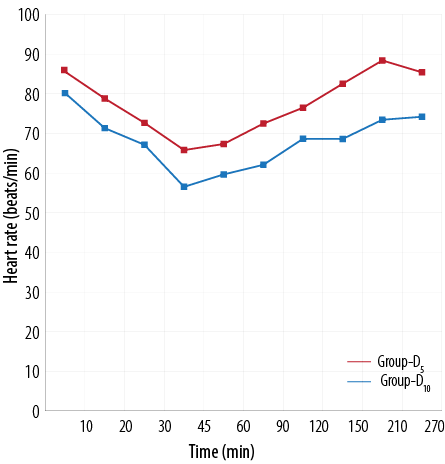
Figure 1: Comparison of heart rate (beats/min) between the two treatment groups.
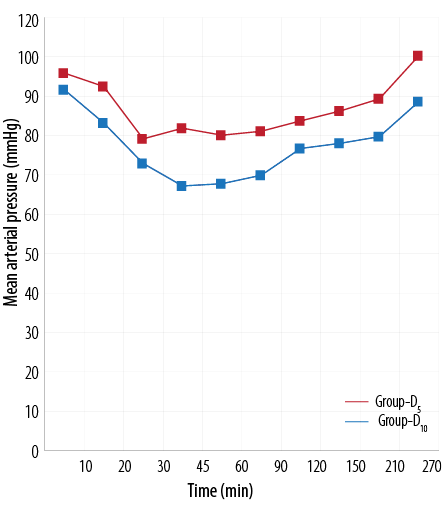
Figure 2: Comparison of mean arterial blood pressure (mmHg) between the two treatment groups.
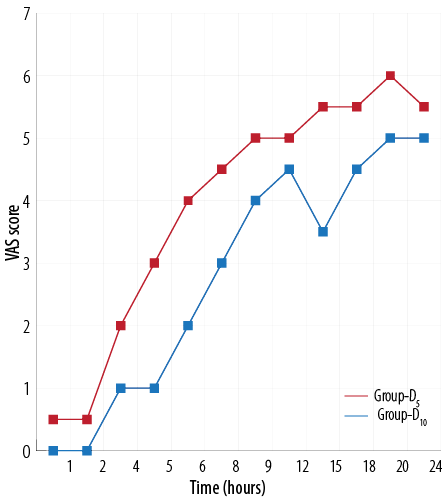
Figure 3: Comparison of visual analogue scale (VAS) score between the two treatment groups.
Discussion
Hysterectomy is the second most common gynecological surgery in the United States after cesarean section.21 Nearly 40% of American women undergo hysterectomy before the age of 60.22 Of the various surgical approaches to hysterectomy (abdominal, vaginal, laparoscopic, or open) the open abdominal approach has been correlated with relatively greater postoperative pain.23
Postoperative pain and its complications can be attenuated with an appropriate perioperative analgesic regimen. Adequate pain relief reduces patients’ anxiety, morbidity, duration of hospitalization, and the associated costs of care.24 Regional anesthesia for major lower abdominal surgeries has long been provided by central neuraxial blockade. Various drugs have been used as adjuvant to intrathecal local anesthetics to achieve quick, dense and prolonged block, but the results were associated with side effects. Dexmedetomidine, a highly selective, α2-adrenergic agonist, has analgesic, sedative, and anesthetic-sparing effects when used in systemic route.25 Use of dexmedetomidine as an adjuvant mixed with local anesthetics has been performed with neuraxial anesthesia in both adult and pediatric patients.17,26
In this prospective, randomized, double-blinded trial on female patients undergoing elective hysterectomies under spinal anesthesia, we compared the effect of 5µg and 10µg of dexmedetomidine added to 15mg 0.5% hyperbaric bupivacaine intrathecally, on the onset time and duration of sensory and motor block as well as the requirement for postoperative rescue analgesic (diclofenac sodium injection) and any associated side effects.
Table 4: Characteristics of sensory and motor blocks between the two study groups.
|
Median sensory block level |
T7 (T7–T10) |
T6 (T6–T9) |
0.071 |
|
Time to sensory blockade T10 (min) |
4.8±0.6 |
4.6±0.1 |
0.130 |
|
Time for sensory regression to T10 (min) |
178.5±23.3 |
259.6±21.4 |
0.009 |
|
Time for sensory regression to S2 (min) |
221.4±22.7 |
297.7±34.1 |
0.001 |
|
Time to maximum motor block of modified Bromage scale 3 (min) |
5.5±0.9 |
5.5±0.9 |
0.060 |
|
Time to motor recovery to modified Bromage scale 0 (min) |
156.0±18.4 |
167.0± 23.8 |
0.023 |
|
Duration of analgesia (min) |
242.2±23.9 |
366.6±24.4 |
0.012 |
Table 5: Comparison of side effects in each group.
|
Nausea |
7 |
9 |
0.551 |
|
Vomiting |
0 |
2 |
0.240 |
|
Shivering |
12 |
16 |
0.352 |
|
Bradycardia (HR<60bpm) |
22 |
27 |
0.092 |
|
Bradycardia (HR<50bpm)* |
6 |
14 |
0.030 |
|
Hypotension (SBP<100mm Hg) |
16 |
20 |
0.284 |
|
HR: heart rate; SBP; systolic blood pressure
*atropine required; **mephenteramine required |
The demographic profile, indications, and hemodynamic parameters of the two groups, which were statistically insignificant, was similar with other research investigations and provided us a uniform platform to compare the results obtained. A similar study on the role of dexmedetomidine for postoperative analgesia, also conducted with 100 patients, yielded similar results.19
The median height of sensory blockade in group D10 produced one segment higher block than the group D5 but the comparison is clinically and statistically insignificant (p>0.050). Similarly, Gupta et al,19 found that the addition of 5µg dexmedetomidine to intrathecal ropivacaine produced one segment higher sensory block than placebo and the results were clinically insignificant.
The median time taken to achieve T10 level sensory block was earlier in the D10 group than the D5 group (4.6 min and 4.8 min, respectively). Similarly, time for maximum motor block of modified Bromage scale 3 was earlier in the D10 group than the D5 group (5.46 min and 5.54 min, respectively). However, in neither case were we able to demonstrate statistical significance. Although our results were similar to that of Esmaoğlu et al,27 who found the onset of sensory and motor blocks was earlier in dexmedetomidine group, their results were statistically significant. Another study also found that dexmedetomidine produced earlier onset of sensory and motor block in a dose-dependent manner for patients with urological operations.18 In our study, sensory regression time to T10 and S2 were significantly delayed in the D10 group compared to the D5 group (259.6 min vs. 178.5 min and 297.7 min vs. 221.35 min, respectively) which means increased dexmedetomidine in the subarachnoid space produced a more sustained sensory block. Again, motor block regression to modified Bromage 0 was significantly prolonged in the D10 group compared to theD5 group (167.08 min vs. 156 min, respectively). Gupta et al,19 found that sensory block regression was significantly slower with the addition of intrathecal dexmedetomidine when compared to ropivacaine alone, as both time to two segment regressions and time to S2 regression were significantly delayed with intrathecal dexmedetomidine. Esmaoglu et al,27 also found that addition of dexmedetomidine to levobupivacaine caused a significant delay in sensory as well as motor block regression for the patients undergoing transurethral endoscopic surgery. Eid et al,28 also concluded that 15µg intrathecal dexmedetomidine with bupivacaine significantly prolonged the time to two segment regression, sensory regression to S1 and regression of motor block to modified Bromage 0 in a dose dependent manner compared to the 10µg dexmedetomidine and saline control group. The authors also found that addition of dexmedetomidine intrathecally caused prolongation of first rescue analgesic requirement significantly and this prolongation occured in a dose dependent manner.28
In our study, mean duration of analgesia in the D10 and D5 group was 366.6 min and 242.1 min, respectively (p<0.050)[Table 4]. Gupta et al,19 found that the duration of analgesia was significantly prolonged with the addition of intrathecal dexmedetomidine compared to use of ropivacaine alone intrathecally (478.4±20.9 min and 241.7±21.7 min, respectively). A meta-analysis of use of dexmedetomidine in regional anesthesia stated that the sensory duration, motor blockade and request for rescue analgesia was prolonged in the dexmedetomidine group.29
In our study, the analgesic effect of intrathecal bupivacaine was potentiated in a dose dependent manner by intrathecal adjuvant dexmedetomidine. Patients in the D10 group required significantly less diclofenac sodium injection (121.3mg vs. 221.1mg) in the first 24 hours of the postoperative period than patients in the D5 group (p<0.010). Gupta et al,19observed less diclofenac sodium consumption in intrathecally dexmedetomidine, ropivacaine treated group than ropivacaine (0.9mg vs. 2.7mg) alone. Diclofenac consumption was reduced in a dose dependent manner after intrathecal dexmedetomidine administration in another study.28
Bradycardia was observed in both the D10 and D5 groups (27 vs. 22 patients, respectively) of which 14 patients in D10 group required atropine (0.6mg iv) injection and six patients in the D5 group required active management. This side effect was found to be statistically as well as clinically significant. Other side effects, including hypotension, nausea, vomiting, and shivering, were noted in both the groups but were statistically insignificant. Other authors also observed similar side effects without any significant difference between the two groups.19,27 The occurrence of hypotension and bradycardia in our study was possibly due to the higher dose of dexmedetomidine in D10 group.
Although the mechanism is unclear, the use of intrathecal alpha-2 adrenoceptor agonists like dexmedetomidine prolongs the motor and sensory block duration of local anesthetics. Dexmedetomidine acts by binding to presynaptic C-fibers and postsynaptic dorsal horn neurons. Intrathecal alpha-2 adrenoceptor agonists act by depressing the release of C-fiber transmitters and by hyperpolarizing postsynaptic dorsal horn neurons.30 The antinociceptive effect may explain the prolongation of the sensory block when added to spinal anesthetics. The prolongation of the motor block of spinal anesthetics may result from the binding of alpha-2 adrenoceptor agonists to motor neurons in the dorsal horn.31
Conclusion
The use of 10µg dexmedetomidine compared to 5µg dexmedetomidine to adjuvant hyperbaric bupivacaine 0.5% more efficiently hastens the onset and prolongs the duration of sensory and motor blockade and reduces the requirement of rescue analgesic in the postoperative period in patients undergoing elective abdominal hysterectomy.
Disclosure
The authors declared no conflict of interests. No funding was received for this work.
references
- Rodgers A, Walker N, Schug S, McKee A, Kehlet H, van Zundert A, et al. Reduction of postoperative mortality and morbidity with epidural or spinal anaesthesia: results from overview of randomised trials. BMJ 2000 Dec;321(7275):1493.
- Borendal Wodlin N, Nilsson L, Carlsson P, Kjolhede P. Cost-effectiveness of general anesthesia vs spinal anesthesia in fast-track abdominal benign hysterectomy. Am J Obstet Gynecol 2011 Oct;205(4):326.e1-7.
- Wilder-Smith OH, Arendt-Nielsen L, Gäumann D, Tassonyi E, Rifat KR. Sensory changes and pain after abdominal hysterectomy: a comparison of anesthetic supplementation with fentanyl versus magnesium or ketamine. Anesth Analg 1998 Jan;86(1):95-101.
- Durant PA, Yaksh TL. Drug effects on urinary bladder tone during spinal morphine-induced inhibition of the micturition reflex in unanesthetized rats. Anesthesiology 1988 Mar;68(3):325-334.
- Chinachoti T, Nilrat P, Samarnpiboonphol P. Nausea, vomiting and pruritus induced by intrathecal morphine. J Med Assoc Thai 2013 May;96(5):589-594.
- Sultan P, Gutierrez MC, Carvalho B. Neuraxial morphine and respiratory depression: finding the right balance. Drugs 2011 Oct;71(14):1807-1819.
- Karaman S, Kocabas S, Uyar M, Zincircioglu C, Firat V. Intrathecal morphine: effects on perioperative hemodynamics, postoperative analgesia, and stress response for total abdominal hysterectomy. Adv Ther 2006 Mar-Apr;23(2):295-306.
- de Oliveira GS Jr, Balliu B, Nader A, McCarthy RJ. Dose-ranging effects of intrathecal epinephrine on anesthesia/analgesia: a meta-analysis and metaregression of randomized controlled trials. Reg Anesth Pain Med 2012 Jul-Aug;37(4):423-432.
- Lauretti GR, Mattos AL, Gomes JM, Pereira NL. Postoperative analgesia and antiemetic efficacy after intrathecal neostigmine in patients undergoing abdominal hysterectomy during spinal anesthesia. Reg Anesth 1997 Nov-Dec;22(6):527-533.
- Nath MP, Garg R, Talukdar T, Choudhary D, Chakrabarty A. To evaluate the efficacy of intrathecal magnesium sulphate for hysterectomy under subarachnoid block with bupivacaine and fentanyl: A prospective randomized double blind clinical trial. Saudi J Anaesth 2012 Jul;6(3):254-258.
- Kim MH, Lee YM. Intrathecal midazolam increases the analgesic effects of spinal blockade with bupivacaine in patients undergoing haemorrhoidectomy. Br J Anaesth 2001 Jan;86(1):77-79.
- Khezri MB, Ghasemi J, Mohammadi N. Evaluation of the analgesic effect of ketamine as an additive to intrathecal bupivacaine in patients undergoing cesarean section. Acta Anaesthesiol Taiwan 2013 Dec;51(4):155-160.
- Engelman E, Marsala C. Efficacy of adding clonidine to intrathecal morphine in acute postoperative pain: meta-analysis. Br J Anaesth 2013 Jan;110(1):21-27.
- Reves JG, Glass P. Intravenous anesthetics. In: Miller RD, Eriksson LI, Fleisher LA, Wiener-Kronish GP, Young WL, editors. Miller’s Anesthesia. 7th ed. Philadelphia, PA: Elsevier Churchill Livingstone; 2010. p. 756-767.
- Jorm CM, Stamford JA. Actions of the hypnotic anaesthetic, dexmedetomidine, on noradrenaline release and cell firing in rat locus coeruleus slices. Br J Anaesth 1993 Sep;71(3):447-449.
- Kanazi GE, Aouad MT, Jabbour-Khoury SI, Al Jazzar MD, Alameddine MM, Al-Yaman R, et al. Effect of low-dose dexmedetomidine or clonidine on the characteristics of bupivacaine spinal block. Acta Anaesthesiol Scand 2006 Feb;50(2):222-227.
- Al-Ghanem SM, Massad IM, Al-Mustafa MM, Al-Zaben KR, Qudaisat IY, Qatawneh AM, et al. Effect of adding dexmedetomidine versus fentanyl to intrathecal bupivacaine on spinal block characteristics in gynecological procedures: A double blind controlled study. Am J Appl Sci 2009;6:882-887.
- Al-Mustafa MM, Abu-Halaweh SA, Aloweidi AS, Murshidi MM, Ammari BA, Awwad ZM, et al. Effect of dexmedetomidine added to spinal bupivacaine for urological procedures. Saudi Med J 2009 Mar;30(3):365-370.
- Gupta R, Bogra J, Verma R, Kohli M, Kushwaha JK, Kumar S. Dexmedetomidine as an intrathecal adjuvant for postoperative analgesia. Indian J Anaesth 2011 Jul;55(4):347-351.
- Kim JE, Kim NY, Lee HS, Kil HK. Effects of intrathecal dexmedetomidine on low-dose bupivacaine spinal anesthesia in elderly patients undergoing transurethral prostatectomy. Biol Pharm Bull 2013;36(6):959-965.
- Graves EJ. Detailed diagnoses and procedures, National Hospital Discharge Survey, 1993. Vital Health Stat 13 1995 Oct;13(122):1-288.
- Wilcox LS, Koonin LM, Pokras R, Strauss LT, Xia Z, Peterson HB. Hysterectomy in the United States, 1988-1990. Obstet Gynecol 1994 Apr;83(4):549-555.
- Hwang JL, Seow KM, Tsai YL, Huang LW, Hsieh BC, Lee C. Comparative study of vaginal, laparoscopically assisted vaginal and abdominal hysterectomies for uterine myoma larger than 6 cm in diameter or uterus weighing at least 450 g: a prospective randomized study. Acta Obstet Gynecol Scand 2002 Dec;81(12):1132-1138.
- Parvizi J, Miller AG, Gandhi K. Multimodal pain management after total joint arthroplasty. J Bone Joint Surg Am 2011 Jun;93(11):1075-1084.
- Huang R, Hertz L. Receptor subtype and dose dependence of dexmedetomidine-induced accumulation of [14C]glutamine in astrocytes suggests glial involvement in its hypnotic-sedative and anesthetic-sparing effects. Brain Res 2000 Aug;873(2):297-301.
- El-Hennawy AM, Abd-Elwahab AM, Abd-Elmaksoud AM, El-Ozairy HS, Boulis SR. Addition of clonidine or dexmedetomidine to bupivacaine prolongs caudal analgesia in children. Br J Anaesth 2009 Aug;103(2):268-274.
- Esmaoğlu A, Türk S, Bayram A, Akın A, Uğur F, Ulgey A. The effects of dexmedetomidine added to spinal levobupivacaine for transurethral endoscopic surgery. Balkan Med J 2013 Jun;30(2):186-190.
- Eid HE, Shafie MA, Youssef HY. Dose-related prolongation of hyperbaric bupivacaine spinal anesthesia by dexmedetomidine. ASJA 2011 Jul;4(2):83-95.
- Abdallah FW, Brull R. Facilitatory effects of perineural dexmedetomidine on neuraxial and peripheral nerve block: a systematic review and meta-analysis. Br J Anaesth 2013 Jun;110(6):915-925.
- Eisenach JC, De Kock M, Klimscha W. α(2)-adrenergic agonists for regional anesthesia. A clinical review of clonidine (1984-1995). Anesthesiology 1996 Sep;85(3):655-674.
- Harada Y, Nishioka K, Kitahata LM, Kishikawa K, Collins JG. Visceral antinociceptive effects of spinal clonidine combined with morphine, [D-Pen2, D-Pen5] enkephalin, or U50,488H. Anesthesiology 1995 Aug;83(2):344-352.