Pulmonary Alveolar Microlithiasis (PAM) is a rare disease of unknown etiology, characterized by the formation and accumulation of laminated calcispherites in the alveoli.1-3 However, its exact pathogenesis is still unknown. The clinical features may vary and patients may be asymptomatic for a long period of time with subsequent occurrence of dyspnea, dry cough, and chest pain, leading to cor pulmonale.4,5 Spontaneous pneumothorax may occur years after the diagnosis.6
In this article, the authors present a case of PAM in a 43-year-old man with spontaneous pneumothorax without previous respiratory symptoms.
Case Report
A 43-year-old male, non-smoker, presented to Tohid Hospital, Sanandaj, Iran, with sudden onset shortness of breath and right-sided chest pain, which was sharp and aggravated by deep inspiration. The symptoms started two days after a common cold. The patient was entirely asymptomatic and denied dyspnea, cough, hemoptysis, and weight loss before recent presentation. The patient had no family history of pulmonary disease, took no medications, and had regular daily exercises without difficulty.
On physical examination, the patient was anxious and tachypneic. Pulmonary auscultation revealed reduced breath sounds in the upper region of the right hemithorax and inspiratory crackles in lower third of both lung fields. Cardiac auscultation revealed normal heart sounds and the patient had no cyanosis, or observable peripheral edema.
Blood tests showed normal values for complete blood counts and serum chemistries and the patient’s arterial oxygen saturation, without the use of supplemental oxygen, was 81%.
Plain chest films showed right-sided pneumothorax and extensive bilateral dense opacities most marked in the middle and lower zones [Figure 1]. In addition, computerized tomography (CT) examination on the admission day revealed right-sided pneumothorax and high attenuation of lung parenchyma.
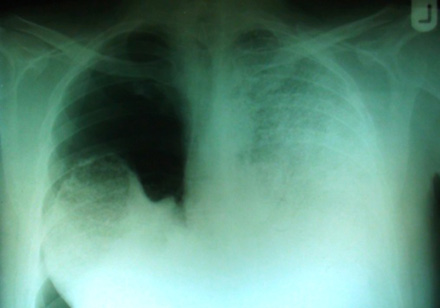
Figure 1: The chest plain film taken on admission revealed right side pneumothorax, extensive bilateral dense opacities obliterated the cardiac and mediastinal borders.
After insertion of a chest tube in the right side of the patient’s chest, on the second day of admission, the dyspnea subsided and repeated chest radiography revealed almost complete re-expansion of the right lung. Following chest tube removal, the patient was discharged on day four. Two weeks later, high resolution CT (HRCT) of lungs and pulmonary function tests were performed. HRCT of the lungs showed high attenuation, increasing from the apex to the lung base, ground glass opacities, and sub-pleural cysts [Figure 2]. Calcification along the interlobular septa and sub-pleural calcifications were also noticed [Figure 3]. Spirometry results were normal with forced vital capacity (FVC) and forced expiratory volume in 1 second (FEV1) more than 80% of the predicted values.
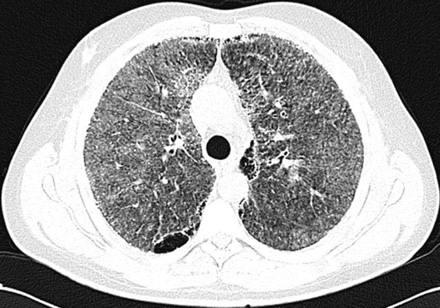
Figure 2: High-resolution computerized tomography of lungs reveals ground glass attenuation, septal thickening, and sub-pleural cysts.
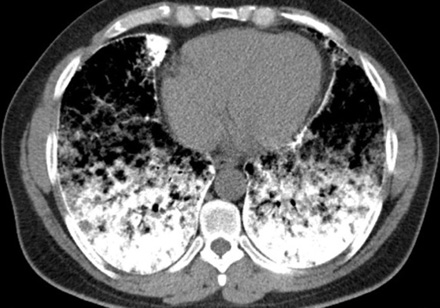
Figure 3: Chest computerized tomography scan of lower zone shows greater involvement: dens calcification of interlobular septa, sub-pleural zone, and pericardial layer.
The patient underwent transbronchial lung biopsy during the course of diagnostic fiber-optic bronchoscopy. Histopathologic examination revealed interstitial inflammatory cell infiltration in conjunction with areas of fibrosis. Multiple laminated calcispherites were found in the alveoli [Figure 4]. These findings were suggestive of PAM.
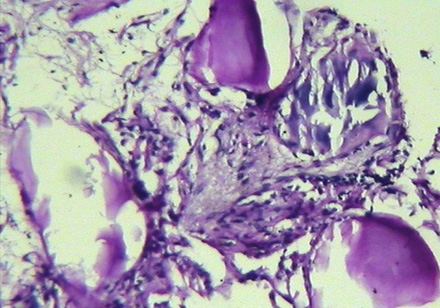
Figure 4: Histopathologic examination of lung shows interstitial infiltration by inflammatory cells, areas of fibrosis and laminated calcispherites within the alveoli.
Discussion
PAM was first described by Malpighi in 1686, who provideda concise and precise macroscopic description of the disease.7 In 1918, Harbitz provided an accurate autopsy of a second case but it was Puhr in 1933 who named the disease mikrolithiasis alveolaris pulmonum.8,9
A review of 576 published cases showed that there was no gender predominance and the ages of affected patients ranged from newborn to those in their ninth decade of life .10
The most accepted etiology for PAM is that it is an inherited metabolic abnormality, involving the enzyme carbonic anhydrase, which promotes alkalinity of the alveolar surface and consequent precipitation of calcareous salt.11 Furthermore, familial incidence is common, particularly in siblings. A family history for the disease was found in one-third of patients.1 Inactivating mutations in the Scl-34A2 gene have been reported in familial cases of pulmonary alveolar microlithiasis. The Scl-34A2 gene encodes a type IIb sodium phosphate cotransporter that is expressed in type II alveolar cells.12
There is a striking discordance between the extent of radiologic involvement and the severity of clinical presentation in PAM patients. Patients may remain asymptomatic for many years, and the condition may progress slowly leading to progressive dyspnea and ultimately result in cor pulmonale. Other reported symptoms are cough, fatigue, chest pain, hemoptysis, palpitations, and headache.11-13 Recurrent pneumothoraces may occur over the course of the disease. Shishido et al14 reported a case of PAM in a patient who developed pneumothorax 34 years after the diagnosis was established. Timothy et al,15 described the surgical management of recurrent pneumothoraces in a case of PAM, decades after initial diagnosis. It is believed that progression of emphysematous bullae resulting in spontaneous pneumothorax occurs in the chronic phase of PAM but pneumothorax symptoms were the first presentation of the disease in our patient, which has not been reported in previous studies.
Although pulmonary function tests may initially yield normal results, restrictive pattern is a common finding during the course of the disease.7 Our case, however, showed a normal pattern of pulmonary function studies after re-expansion of the lung.
The characteristic feature of PAM on the chest radiograph revealed a picture of infiltrates as fine sand-like calcific micronodules (“sandstorm lung”) diffusely in both lungs.16 This feature resembled the radiographic finding of calcified miliary tuberculosis,17 small thin-walled subpleural cysts are described. In addition, which are responsible for “black pleura” sign seen in chest X-rays.3,5,6
As a diagnostic method, HRCT of the chest has greater sensitivity than a chest X-ray. HRCT findings in patients with PAM vary considerably, nevertheless, the most common findings are ground glass opacities and diffuse calcified nodules.5,6,10,16 Others include linear calcifications, calcification of interlobular septa, mosaic pattern of attenuation, small calcipherites within the thickened pleura, crazy paving pattern, and calcifications along the heart borders.3-7 The latter was seen in our patient. In addition, there were visible apical cysts on both sides. These cysts were ribbon like and arranged along the mediastinal and parietal pleura, and could be the source of spontaneous pneumothorax in our case. Whole body 99mTechenetium methylen diphosphate scintigraphy may show diffusely increased radiotracer uptake over the lungs.18
There is no known medical treatment to reduce or halt the progression of PAM. Lung transplantation remains the only possible treatment for end-stage cases.4,19 Recurrent pneumothorax in PAM should be managed with thoracotomy and excision of affected areas of lung, and subsequent lung defects should be oversewn and buttressed.15
Conclusion
In conclusion, PAM is a rare, chronic lung disease characterized by progressive clinical course. Spontaneous recurrent pneumothorax may occur in the later phase of the disease but it should be highlighted that the first presentation of a PAM case could be the occurrence of pneumothorax.
Disclosure
The author declared no conflict of interest. No funding was received for this work.
references
- Emri S, Cöplü L, Selçuk ZT, Sahin AA, Baris YI. Hypertrophic pulmonary osteoarthropathy in a patient with pulmonary alveolar microlithiasis. Thorax 1991 Feb;46(2):145-146.
- Thapa R, Ganguly D, Ghosh A. Pulmonary alveolar microlithiasis in siblings. Indian Pediatr 2008 Feb;45(2):154-156.
- Abdalla G, Marchiori E, Zanetti G, Mucillo A, Pereira MA, Ventura N, et al. Pulmonary Alveolar Microlithiasis: A case report with emphasis on imaging findings.Case Reports in Medicine [Internet].2010 [cited 10 Jan 2013]; Article ID 819242:[about 4p.]. Available at http://www.hindawi.com/journals/crim/2010/819242/.
- Samano MN, Waisberg DR, Canzian M, Campos SV, Pêgo-Fernandes PM, Jatene FB. Lung transplantation for pulmonary alveolar microlithiasis: a case report. Clinics (Sao Paulo) 2010 Feb;65(2):233-236.
- Marchiori E, Gonçalves CM, Escuissato DL, Teixeira KI, Rodrigues R, Barreto MM, et al. Pulmonary alveolar microlithiasis: high-resolution computed tomography findings in 10 patients. J Bras Pneumol 2007 Sep-Oct;33(5):552-557.
- Korn MA, Schurawitzki H, Klepetko W, Burghuber OC. Pulmonary alveolar microlithiasis: findings on high-resolution CT. AJR Am J Roentgenol 1992 May;158(5):981-982.
- Malhotra B, Sabharwal R, Singh M, Singh A. Pulmonary alveolar microlithiasis with calcified pleural plaques. Lung India 2010 Oct;27(4):250-252.
- Harbitz F. Extensive calcification of the lungs as a distinct disease. Arch Intern Med 1918;21(1):139-146 .
- Puhr L. Mikrolithiasis alveolaris pulmonum (Pulmonary alveolar microlithiasis). Virchos Arch (Pathol Anat) 1933;290:156.
- Mariotta S, Ricci A, Papale M, De Clementi F, Sposato B, Guidi L, et al. Pulmonary alveolar microlithiasis: report on 576 cases published in the literature. Sarcoidosis Vasc Diffuse Lung Dis 2004 Oct;21(3):173-181.
- O’Neill RP, Cohn JE, Pellegrino ED. Pulmonary alveolar microlithiasis–a family study. Ann Intern Med 1967 Nov;67(5):957-967.
- Jönsson AL, Simonsen U, Hilberg O, Bendstrup E. Pulmonary alveolar microlithiasis: two case reports and review of the literature. Eur Respir Rev 2012 Sep;21(125):249-256.
- Gayathri Devi HJ, Mohan Rao KN, Prathima KM, Das JK. Pulmonary alveolar microlithiasis. Lung India 2011 Apr;28(2):139-141.
- Shishido S, Toritani T, Nakano H, Tokushima T. [A case of alveolar microlithiasis which developed spontaneous pneumothorax due to progression of emphysematous bullae during 34 years after established diagnosis]. Nihon Kyobu Shikkan Gakkai Zasshi 1993 Jul;31(7):881-885.
- Batchelor TJ, Ngaage DL, McGivern DV, Cowen ME. Surgical management of pneumothorax in pulmonary alveolar microlithiasis. Ann Thorac Surg 2007 Jul;84(1):290-292.
- Castellana G, Lamorgese V. Pulmonary alveolar microlithiasis. World cases and review of the literature. Respiration 2003 Sep-Oct;70(5):549-555.
- Gayathri Devi HJ, Mohan Rao KN, Prathima KM. Pulmonary Alveolar Microlithiasis. Lung India 2011;28(2):139-141 .
- Glynos C, Papathanasiou N, Nikoloutsou I, Stathopoulos GT. Pulmonary alveolar microlithiasis. Am J Respir Crit Care Med 2011 Sep;184(6):740.
- Siddiqui NA, Fuhrman CR. Best cases from the AFIP: Pulmonary alveolar microlithiasis. Radiographics 2011 Mar-Apr;31(2):585-590.