| |
Abstract
Objective: This study aims to determine the relationship between body size and body shape with the risk of breast cancer.
Methods: In this case control study, 480 women participated (240 women with breast cancer in case group and 240 healthy women in control group). After completing the interview form, the weight, height, waist circumference, hip circumference and breast size, were measured. The data were analyzed using statistical test by SPSS11.5.
Results: The present study showed that the mean of hip circumference were significantly different in both groups (p=0.036). The size of the breast was statistically significant between the two groups. Thyroid type, one of the body shapes, was more seen in the case group than control group (p<0.001).
Conclusion: This study revealed that the risk of breast cancer increases with increased hip circumference. In addition, the results indicate that body shape may be a useful predictor in determining the risk of breast cancer. More studies should be designed to address this subject.
Keywords: Breast cancer; Body size; Waist circumference; Waist-hip ratio.
Introduction
Breast cancer is the most frequent malignancies in women,1 and there has also been an increasing expression of breast cancer in the developing countries.2 In Iran, breast cancer ranks first among common cancers diagnosed in women, with 21.4% of all malignancies in women being attributed to breast cancer.3 It is therefore critical to offer a quick diagnosis in suspected cases.1 It has been suggested that body size plays a major role in the increasing rate of breast cancer.4 Indices such as height, weight, body mass index, waist circumference, the ratio of waist-to-hip circumference and weight changes are regarded as effective factors for breast cancer.4 In Iran, the prevalence of overweight and obesity was common among 57% of women in 2005 and it is predicted to reach 74% by 2015. Abdominal obesity was shown to be between 55% and 63% in Iranian women.5 Although the characteristics of the body are introduced as the probable risk indicators of breast cancer, study findings on the correlation between obesity and the risk of breast cancer in Western women are somewhat different.6 Nemesure and colleagues suggested that body mass index in women of postmenopausal phase is positively correlated with breast cancer, while the premenopausal phase is negatively correlated with breast cancer.4 The results of a study showed that women in the obese range had an increased risk of breast cancer.6
Agurs-Collins and colleagues indicated no significant relation between body mass index and breast cancer.7 An investigation from India indicated that increased body mass index, waist circumference and hip circumference were risk factors for breast cancer both in pre- and postmenopausal women.8 Based on another report, waist circumference may be postulated to affect the risk of breast cancer, assuming that the changes are due to hormone replacement therapy (HRT).9 In the study conducted by Montazeri et al. women with breast cancer were notably shorter compared to their counterparts in the control group.6 In addition, results from the survey suggested a reduced risk of breast cancer in short women compared with taller women.6 However, in a study conducted in Italy, Tavani and colleagues were able to demonstrate that breast cancer risk is not substantially associated with the tallest women compared with the shortest.10
On the other hand, it does appear that body type is positively correlated with the risk of breast cancer. Body type is mainly affected by two factors, namely; heredity and lifestyle.11 Body type is categorized into four based on the type and amount of body fat distribution and waist circumference, hip circumference and breast size, these are: Android, Gynacoid, Thyroid and Lymphatic.12 In the review of Tehard et al, it was reported that women with the android body type were at a greater risk of breast cancer in menopausal period,13 but studies have rarely reported this fact in Iran.
The correlation of breast size and breast cancer risk is, however, controversial14 Manab et al., stated that adipose tissue lead to the development of breast carcinoma cells.15 Scitt et al. also showed that breast cancer patients had asymmetric and larger sized breasts vs. healthy women of the same age.2 Another study suggested that young women with large breasts were at an increased risk of advanced breast cancer during their premenopausal period. Koch et al. and Tavani et al. found no correlation between breast size and the risk of breast cancer.14,16 However, oncologists suggest that a relationship between large breast size and greater risk of breast cancer does actually exist, but this hypothesis requires further studies in order to be verified.14
Breast volume augmentation with silicone implants have shown an inexplicable reduction in breast cancer.14 Moreover, Brinton et al. reported that the use of breast implants had no impact on the risk of breast cancer,17 while Brawn et al. were able to demonstrate that the risk of breast cancer had significantly decreased after breast reduction through mammoplasty operation.18 In view of the conflicting results from various studies and the increasing risk of breast cancer worldwide, this study was thus conducted to investigate the correlation of body type and size with regards to breast cancer risk.
Methods
This study was officially approved by Mashhad University of Medical Sciences, which referred researchers to the Omid and Ghaem hospitals in Mashhad, where convenience sampling began. The sampling was accomplished between November 2010 and September 2011. Informed consent was given by all participants who were briefed about the aims and method of the research project. Each participant completed a research unit election form and the eligible candidates were interviewed in order to be enrolled into the study.
In this case control study, the samples in case group included the women with in-situ or metastatic breast cancer whose disease had been confirmed through histological examinations. The medical profiles of each participant were used to confirm the cancer. Another group of healthy participants included women with normal findings upon examination and no history of cancer, but were referred for routine check-up.
A structured questionnaire was completed by the researcher about the selection of specimens. A scale was set on a flat surface for measuring weight (ensuring the accuracy of the scale by using a 1 kg standard weight). The women were weighed with light clothes and no shoes. Height was measured by participants standing on a flat surface in front of a wall, not wearing shoes, with their insteps, hips, shoulders and occipital bones attached to the wall. A flat device (for instance a ruler) was placed on the head and using a tape meter which had been installed on the wall beforehand, the measurements were recorded with the accuracy of decimeter. Waist circumference was measured with the participants standing flat and having the distance between the lowest rib margin and iliac crest measured with a tape meter. The waist circumference was estimated after expiration of the obtained middle point. Hip circumference was measured using a flexible meter at its widest section.
Breast size (without bra) was determined using a flexible tape meter at the largest part of the breast (which is usually around the nipple surface and just beneath the breasts [thoracic cage size]), and the variations were then calculated. Also, the participants gave an impression of their bra size during puberty and the present time, and their responses were recorded in the questionnaire. The symmetry and shape of the breasts were observed and noted in the check list by the researcher. Body mass index and waist-to-hip circumference ratio were also estimated. Body type was determined via a body type questionnaire,12 and recorded in the main questionnaire. Data analysis was done using SPSS11.5 software and statistical tests.
Results
In the current study, the average age was 50 ± 11 in affected women and 46 ± 8 in non-affected women, in which a significant variation was observed (p<0.001). The age of the first and second parturition in the case group were 22 ± 5 and 32 ± 6 years, respectively; and 20 ± 4 and 31 ± 6 years in the control group, respectively. The differences in the age at the first and second parturitions were statistically significant between the two groups (p=0.001). There was a significant difference between the two groups in terms of place of residence, education, type of menstruation, menopause and family history of breast cancer (p<0.05); but in terms of profession, marital status (single, married), age at menarche, income, as well as the number of deliveries and parturitions, no significant variation was observed. (Table 1)
Table 1: Distribution of demographic characteristics and fertility between the case and the control groups.
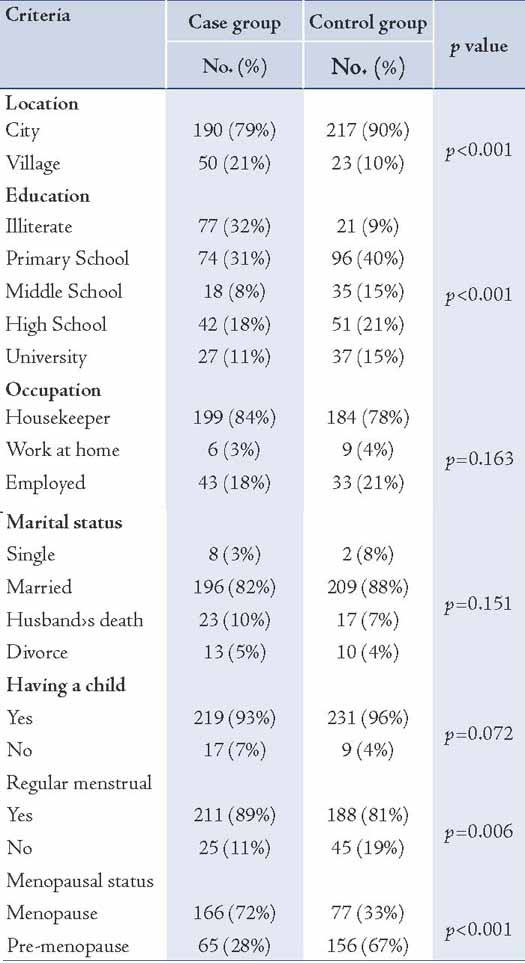
Waist circumference was higher in the case group than the control group, which was not statistically significant (p=0.093). The hip circumference was significantly higher in the case group compared to the control group (p=0.036); moreover, no significant difference was observed between the two groups in the case of the waist to hip circumference ratio (p=0.438). The waist to hip circumference ratio (W/H ≥0.88) was observed in the 94 participants comprising the case group (43%) and the 96 participants comprising the control group (43%), but no significant difference was seen statistically (p=0.511). The breast size (just beneath the breasts) was significantly larger in the case group compared with the control group (p<0.001); however, the breast size at its largest part was significantly smaller in the case group (p=0.025). In view of the differences between the two breast sizes (at the largest point and the size of thoracic cage just beneath the breast), a significant variance was observed statistically (p<0.001). The average sized bra was 70 ± 28 in the patient group and 81 ± 7 in the control group before diagnosis (p<0.001).
While the average estimated weight was 70 ± 5 kg in the patient group and 70 ± 11 kg in the healthy controls, for which no significant correlation was observed statistically (p=0.903; t= 0.12). However, the average height was 156 ± 11 cm and 158 ± 6 cm in the case and control groups, respectively, which exhibited a positive correlation based on statistical tests (p=0.004; t=2.9). Self reporting by participants on weight before disease diagnosis suggested that 57 cases (25%) were obese, 148 cases (66%) were of normal weight and 20 cases (9%) were lean. However, no significant statistical relationship was associated with body mass index between the two groups (p=0.426). (Table 2)
In logistic regression analysis, a based group was determined for independent quality variables. Then the odds ratio for all other groups was measured in relation to the based group. The based group was the first group for the education variables and menopausal status and it was the last group for other qualitative variables. Cancer risk in samples with primary school was 3.1 times more than illiterate. In quantitative variables, the odds ratio in cancer risk is 1.27 times per 1 cm increase in breast size (chest circumference immediately below the breast, the breast circumference in the most massive part of it and the breast circumference and chest circumference). This model showa the relationship between body size and risk of cancer considering the effect of confounding variable such as education, having child and etc. (Table 3)
In the current study, 166 postmenopausal women (68%) were suffering from breast cancer and 77 (32%) were healthy, whereas the figures were 65 cases (30%) and 152 cases (70%) in premenopausal period, respectively. There was no statistical significance in waist circumference, hip circumference, waist to hip circumference ratio, weight and body mass index during premenopausal and postmenopausal periods between the two groups. However, there was a statistically significant difference in breast size (at the largest point and the thoracic cage size just beneath the breasts), and height between the two groups among postmenopausal women (p<0.05). The differences in body size were statistically insignificant among premenopausal women.
Table 2: Comparison of mean indices of body size in case and control groups.
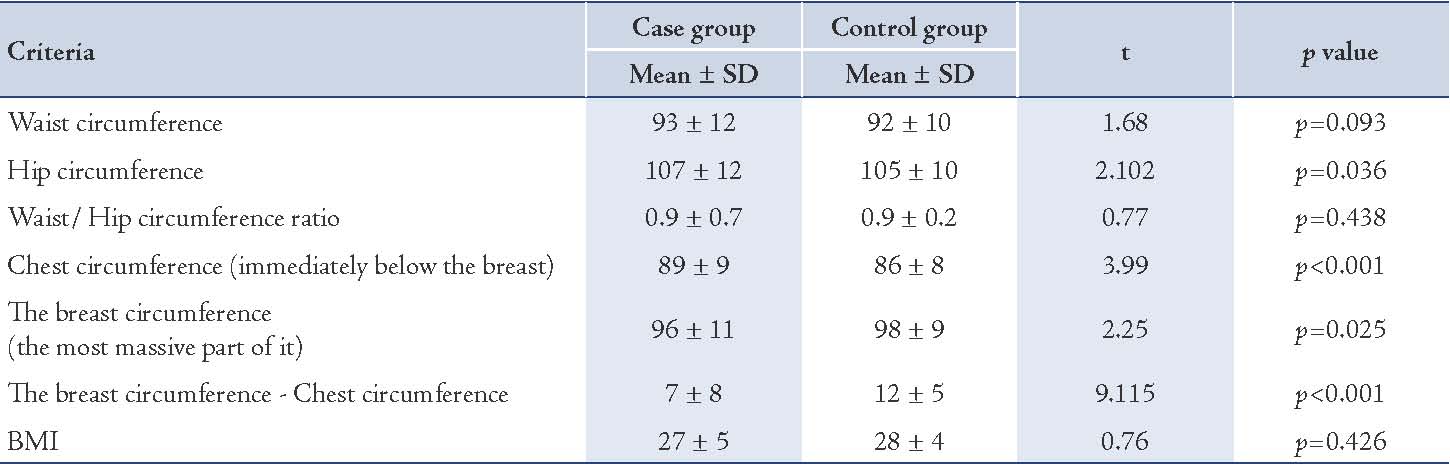
Table 3: Logistic regression analysis of demographic, fertility, and body size variables on breast cancer.
In addition, the thyroid body type was highly expressed in the case group vs. the control group, whilst the other three body types (android, gynecoid and lymphatic) were less common in the case group compared with the control group. These variances were statistically significant between the two groups (p<0.001). (Fig. 1)
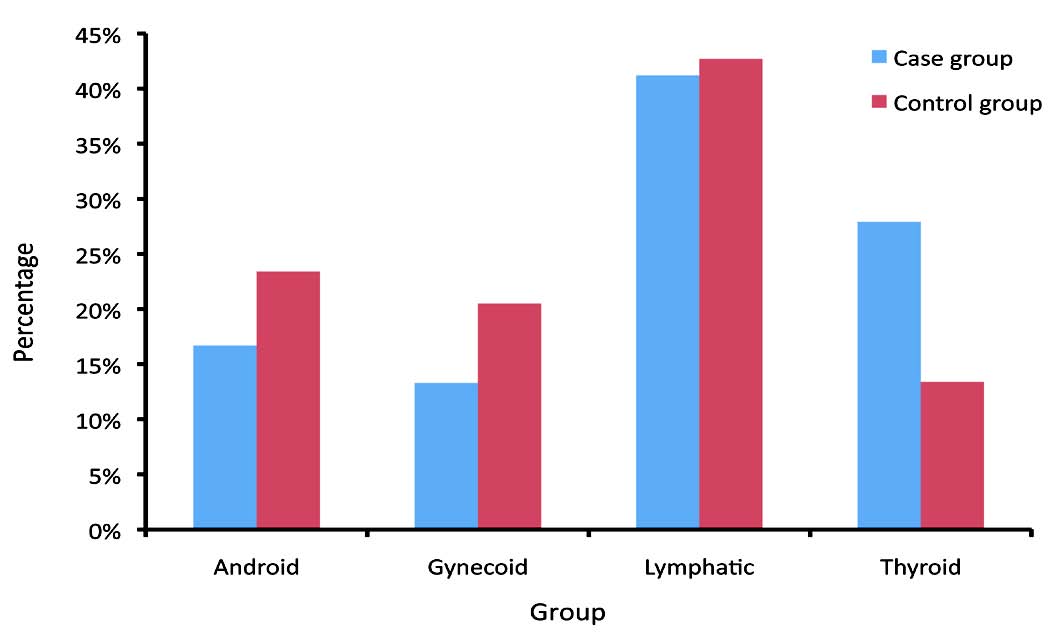
Figure 1: Frequency distribution of body shapes in case and control groups.
Discussion
The present study observed no significant differences in terms of body size variables such as waist circumference and waist to hip circumference ratio between the case group and the control groups, while a significant difference was noted in the average hip circumference between the two groups (p=0.036); however, this difference was not noted when considering the premenopausal and postmenopausal periods independently. Although central fat and height were not measured in this study, several reviews have demonstrated that body in terms of weight and body mass index may be effective against the risk of breast cancer. One study found excessive waist circumference in the postmenopausal period to be associated with breast cancer; however, no such association was observed in the premenopausal period.4 A hypothesis explaining the protective effect in premenopausal women may be the fact that obese women experience amenorrhea or a short secretory phase during menstruation and productivity, which results in decrease in progesterone production. This progesterone reduction may in turn lessen mitosis in mammary cells and cause a subsequent protection against the risk of breast cancer.4
In a study conducted in China, hip circumference was reported to be significantly associated with an increased risk of breast cancer.19 Not surprisingly, the main activity of aromatase which is concomitant with E2 metabolism, is found in hips.19 Furthermore, the advancement of waist and hip circumference has been to be an important factor of breast cancer risk, and is connected with high levels of androgens and insulin, and low levels of sex hormone-binding globulin.19 Increased levels of insulin are associated with advanced risk of breast cancer, and a high waist to hip circumference ratio is an indicator of more fat distribution along the abdominal region, as well as insulin resistance and hyperinsulinemia.20 In addition, increased levels of estradiol have been correlated with increase in weight, hip circumference and waist circumference.21
While epidemiological evidence is less compatible with the correlation between waist-to-hip circumference ratio and breast cancer morbidity,22-26 three prospective studies have reported a positive association between waist-to-hip circumference ratio and breast cancer during postmenopausal period.22-24 However, other prospective and case-control studies have not shown a positive relationship between those variables.22-26 Also, a previous study has suggested that waist to hip circumference ratio is not related to breast cancer risk in premenopausal women and with less compatible findings in postmenopausal women,4 which is in accordance with the present study.
Certainly, the quoted finding of coordination between central fat and breast cancer risk does not mean that there would be no correlation between them, and also poses the notion that anthropometric measures such as waist circumference, hip circumference, waist-to-hip circumference ratio, and body mass index may be ineffective at indicating central fat levels. Applying CT and MRI scans may possibly be more precise in this case, but using these methods can be very costly.26
In the current study, the average height during postmenopausal period in the case group was significantly lower than in the control group (p=0.004), and no significant difference was observed statistically in the premenopausal period, which is in accordance with the findings of Montazeri et al.6 In other studies, no relationship was observed between breast cancer and height.10 However, height is recognized to be a risk factor for breast cancer in white American women, but this relationship was weaker in the premenopausal women.4 Height is also associated with other factors such as nutrition, genetics and hormones, and may therefore impact breast cancer morbidity.4,6 A probable explanation for this may be the fact that insulin-like growth factor is highly expressed to a greater extent in taller women and may therefore act as a predisposing factor for breast cancer risk, but it is important to investigate these prospects further.4
Several biological mechanisms are being considered in order to clarify the manner in which anthropometric factors affect the risk of breast cancer. Obesity may elevate the levels of circulating endogenous sex hormones, insulin and insulin-like growth factors, and thereby decrease the risk of cancer.27 In the present study, the average weight was greater in the case group compared with the control group, but the difference was statistically insignificant (p=0.903). The patients were weighed during the current study, as well as at the time of cancer diagnosis and during treatment, which may not reflect the patients’ real weight before the onset of disease in cases where the patients lost weight due to the cancer. Also, self-reporting of weight before disease onset may be inaccurate as the obese women do not tend to state their real weight compared to their counterparts with normal weight,6 and it is also likely because the disease may not have been recognized during the initial stages and thus weight which might have reported as the normal healthy weight might in fact have been the weight before disease progression, or it could just be that the respondents have no exact knowledge of their real weight.
Several studies have suggested that body size is an important risk factor of breast cancer morbidity.19 Body mass index has been viewed as a factor affecting breast cancer morbidity in both premenopausal and postmenopausal periods,4 in the current study however, no significant difference was observed between the two groups in terms of body mass index (p=0.426). Body mass index has been reported to be inversely correlated with breast cancer morbidity in premenopausal period and is directly related in the postmenopausal period.19
In this study, the average breast size just beneath the breasts was greater in the case group than in the control group with a statistically significant difference (p<0.001). Whereas the mean breast size at its largest part was significantly smaller in the case group compared with the control group (p=0.025), and the mean variance of these two measurements was lower in the case group in comparison to the control group (p<0.001). Therefore, the findings from the current study impart that the average breast size was significantly smaller in the case group rather than the control group (p<0.001). Furthermore, although no relation has been observed between epithelial mass with breast size and breast cancer morbidity, the interplay of adipocytes and breast cancer cells is very clear.14 The reason for the decreased breast mass volume in the case group presented in the current study may have been as a result of decreased weight in patients due to the disease and chemotherapy. On the other hand, however, the participants were questioned on their size of bra before the diagnosis of cancer, which was significantly smaller in the case group compared with the control group (p<0.001).
This article indicates a significant association between body type and breast cancer, as the android, gynecoid and lymphatic body types were highly expressed in the control group and the thyroid body type was most prevalent in the case group (p<0.001). In a prospective study on breast cancer conducted by Kumar et al. (2000), the body fat distribution for the android body type was presented as a critical predictor of patient survival.28 Very few studies have focused on the association of body types with breast cancer risk, with only the gynecoid and android types having been reviewed, in which the body types were indicated by applying the waist circumference, hip circumference and hip-to-waist circumference ratio. In the present study however, four body types (android, gynecoid, lymphatic and thyroid) were evaluated and a specific questionnaire was applied besides the body size measurements, in which nutritional diet, physical activity and personality were analyzed in order to indicate the body type. Nevertheless, anthropometric data may differ in various ethnic and racial groups,29 hence the observed differences between the current study and other investigations may have resulted from environmental and racial differences.
In general, the current observations may suggest diverse effects of types and sizes of the body in relation to breast cancer between different populations, thus further investigations are warranted.
Conclusion
The current study findings highlight an increased risk of breast cancer with increasing hip circumference. Body shape may also be a useful predictor in determing the risk of breast cancer. However, further studies should focus on this subject in different populations.
Acknowledgements
We thank the patients for participating in this study. The authors would like to thank Mashhad University of Medical Sciences for its financial support (Grang No. 89149), administrators at Omid and Ghaem Hospitals (Mashhad, Iran), great appreciation to Ms Bagheri and Mrs Nazari. The authors have no conflicts of interest to declare.
References
1. Lam WW, Chan CP, Chan CF, Mak CC, Chan CF, Chong KW, et al. Factors affecting the palpability of breast lesion by self-examination. Singapore Med J 2008 Mar;49(3):228-232.
2. Scutt D, Manning JT, Whitehouse GH, Leinster SJ, Massey CP. The relationship between breast asymmetry, breast size and the occurrence of breast cancer. Br J Radiol 1997 Oct;70(838):1017-1021.
3. Eftekhari MH, Moradi M. Assessment of Energy and Macronutrients Intake in Breast Cancer Patients. Babol university medical science Journal 2009; 11(3): 60-66.
4. Nemesure B, Wu SY, Hennis A, Leske MC; Barbados National Cancer Study Group. Body size and breast cancer in a black population–the Barbados National Cancer Study. Cancer Causes Control 2009 Apr;20(3):387-394.
5. Veghari G, Sedaghat M, Banihashem S, Moharloei P, Angizeh A, Tazik E, et al. Trends in waist circumference and central obesity in adults, northern iran. Oman Med J 2012 Jan;27(1):50-53.
6. Montazeri A, Sadighi J, Farzadi F, Maftoon F, Vahdaninia M, Ansari M, et al. Weight, height, body mass index and risk of breast cancer in postmenopausal women: a case-control study. BMC Cancer 2008;8:278.
7. Agurs-Collins T, Adams-Campbell LL, Kim KS, Cullen KJ. Insulin-like growth factor-1 and breast cancer risk in postmenopausal African-American women. Cancer Detect Prev 2000;24(3):199-206.
8. Mathew A, Gajalakshmi V, Rajan B, Kanimozhi V, Brennan P, Mathew BS, et al. Anthropometric factors and breast cancer risk among urban and rural women in South India: a multicentric case-control study. Br J Cancer 2008 Jul;99(1):207-213.
9. Sonneschein E, Toniolo P, Terry MB, Bruning PF, Kato I, Koenig KL, et al. Body fat distributiono and obesity in pre and postmenopausal breast cancer. International Epidemiological Association 1999;28:1026-1031 .
10. Tavani A, Braga C, La Vecchia C, Parazzini F, Talamini R, Franceschi S. Height and breast cancer risk. Eur J Cancer 1998 Mar;34(4):543-547.
11. Ziegler RG. Anthropometry and breast cancer. J Nutr 1997 May;127(5)(Suppl):924S-928S.
12. www.liverdoctor.com/Section3/14_bodytypes.asp
13. Tehard B, Clavel-Chapelon F. Several anthropometric measurements and breast cancer risk: results of the E3N cohort study. Int J Obes (Lond) 2006 Jan;30(1):156-163.
14. Koch AD, Nicolai JP, de Vries J. Breast cancer and the role of breast size as a contributory factor. Breast 2004 Aug;13(4):272-275.
15. Manabe Y, Toda S, Miyazaki K, Sugihara H. Mature adipocytes, but not preadipocytes, promote the growth of breast carcinoma cells in collagen gel matrix culture through cancer-stromal cell interactions. J Pathol 2003 Oct;201(2):221-228.
16. Tavani A, Pregnolato A, La Vecchia C, Negri E, Favero A, Franceschi S. Breast size and breast cancer risk. Eur J Cancer Prev 1996 Oct;5(5):337-342.
17. Brinton LA, Lubin JH, Burich MC, Colton T, Brown SL, Hoover RN. Breast cancer following augmentation mammoplasty (United States). Cancer Causes Control 2000 Oct;11(9):819-827.
18. Brown MH, Weinberg M, Chong N, Levine R, Holowaty E. A cohort study of breast cancer risk in breast reduction patients. Plast Reconstr Surg 1999 May;103(6):1674-1681.
19. Wu M-H, Chou Y-C, Yu J-C, Yu C-P, Wu C-C, Chu C-M, et al. Hormonal and body-size factors in relation to breast cancer risk: a prospective study of 11,889 women in a low-incidence area. Ann Epidemiol 2006 Mar;16(3):223-229.
20. Borugian MJ, Sheps SB, Kim-Sing Ch, Olivotto IA, Van Patten C, Dunn BP, et al. Waist-to-hip ratio and breast cancer mortality. Am J Epidemiol 2003 Nov;158(10):963-968.
21. Boyapati SM, Shu XO, Gao YT, Dai Q, Yu H, Cheng JR, et al. Correlation of blood sex steroid hormones with body size, body fat distribution, and other known risk factors for breast cancer in post-menopausal Chinese women. Cancer Causes Control 2004 Apr;15(3):305-311.
22. Folsom AR, Kaye SA, Prineas RJ, Potter JD, Gapstur SM, Wallace RB. Increased incidence of carcinoma of the breast associated with abdominal adiposity in postmenopausal women. Am J Epidemiol 1990 May;131(5):794-803.
23. Kaaks R, Van Noord PA, Den Tonkelaar I, Peeters PH, Riboli E, Grobbee DE. Breast-cancer incidence in relation to height, weight and body-fat distribution in the Dutch "DOM" cohort. Int J Cancer 1998 May;76(5):647-651.
24. Huang Z, Willett WC, Colditz GA, Hunter DJ, Manson JE, Rosner B, et al. Waist circumference, waist:hip ratio, and risk of breast cancer in the Nurses’ Health Study. Am J Epidemiol 1999 Dec;150(12):1316-1324.
25. Muti P, Stanulla M, Micheli A, Krogh V, Freudenheim JL, Yang J, et al. Markers of insulin resistance and sex steroid hormone activity in relation to breast cancer risk: a prospective analysis of abdominal adiposity, sebum production, and hirsutism (Italy). Cancer Causes Control 2000 Sep;11(8):721-730.
26. Morimoto LM, White E, Chen Z, Chlebowski RT, Hays J, Kuller L, et al. Obesity, body size, and risk of postmenopausal breast cancer: the Women’s Health Initiative (United States). Cancer Causes Control 2002 Oct;13(8):741-751.
27. Mathew A, Gajalakshmi V, Rajan B, Kanimozhi V, Brennan P, Mathew BS, et al. Anthropometric factors and breast cancer risk among urban and rural women in South India: a multicentric case-control study. Br J Cancer 2008 Jul;99(1):207-213.
28. Kumar NB, Cantor A, Allen K, Cox CE. Android obesity at diagnosis and breast carcinoma survival: Evaluation of the effects of anthropometric variables at diagnosis, including body composition and body fat distribution and weight gain during life span,and survival from breast carcinoma. Cancer 2000 Jun;88(12):2751-2757.
29. Vasanthakumar P, Kumar P, Rao M. Anthropometric analysis of palpebral fissure dimensions and its position in South Indian ethnic adults. Oman Med J 2013 Jan;28(1):26-32.
|
|