|
Abstract
Objective: Published studies on the prevalence of celiac disease in type 1 diabetes mellitus from the Arab World are scant. We aim to report the prevalence of celiac disease in Omani children with type 1 diabetes mellitus.
Methods: Children with type 1 diabetes mellitus were prospectively screened for celiac disease, at Sultan Qaboos University Hospital, Muscat, Oman over a period of one year (June 2011 - May 2012). Serum anti tissue transglutaminase IgA, endomysial IgA antibodies and total IgA were measured for screening of celiac disease. Children with positive anti-tissue transglutaminase and/or endomysial IgA antibodies underwent endoscopy.
Results: A total of 103 children with type 1 diabetes mellitus were initially included. Ten patients were lost to follow up. Ninety-three patients aged 2-17 years underwent screening for celiac disease. Sixteen patients had positive anti-tissue transglutaminase (17%). Fourteen patients underwent endoscopy with duodenal biopsies, while two were lost to follow-up. Five patients with positive anti-tissue transglutaminase had intestinal biopsy proven celiac disease. The prevalence of celiac disease is 5.5% in our cohort of children and adolescents with type 1 diabetes mellitus.
Conclusions: The prevalence of celiac disease in Omani children and adolescents with type 1 diabetes mellitus is similar to the World’s reported prevalence, but is less than that reported for Middle Eastern Arab children. To our knowledge, this is the first reported study on the prevalence of celiac disease in Omani children with type 1 diabetes mellitus.
Keywords: Celiac Disease; Type 1 Diabetes Mellitus; Anti-tissue transglutaminase; Oman.
Introduction
Celiac Disease (CD) is an immune-mediated disorder evoked by gluten and related prolamines in genetically susceptible individuals. It is characterized by the presence of a variety of non-specific signs and symptoms, enteropathy, CD-specific antibodies, and human leukocyte antigen (HLA)-DQ2 or HLA-DQ8 haplotypes. CD-specific antibodies comprise autoantibodies against tissue transglutaminase (anti-tTG), endomysial antibodies (EMA), and antibodies against deamidated forms of gliadin peptides (DGP).1
The negative impact of CD on health such as growth failure, osteoporosis and intestinal lymphoma may be reduced with dietary treatment.2,3 Early diagnosis and prompt management of CD is, therefore, important in reducing both its morbidity and mortality.4 It is important to diagnose CD not only in children with obvious signs and symptoms but also in children with subtle clinical manifestations and in those who are at risk of developing CD. High prevalence of CD has been reported in patients with type 1 diabetes mellitus (T1DM) with reported prevalence rates ranging from 1-16% in children with T1DM in screening studies published all over the world.5-12
The association between CD and T1DM has been evaluated in several studies. Some studies have reported the prevalence of CD in T1DM to be 20 times more frequent compared to the general population.13,14 CD has also been shown to be more prevalent in the first-degree relatives of T1DM patients.15 Patients with T1DM may lack typical symptoms of CD, and late complications of CD (e.g., intestinal lymphoma) may develop if left undiagnosed. Thus, screening for CD in patients with T1DM seems justified especially due to the high prevalence of CD in this population.16
Data on CD prevalence in Oman is limited.17-19 No reports are available documenting the prevalence of CD in Omani children with T1DM. Published studies on the prevalence of CD in T1DM from the Arab world are few.5-10 The prevalence in the reported studies from Saudi Arabia,5-7 Tunisia,8 West Algeria9 and Libya10 ranges between 4.9 and 11.3%. There is limited data on the prevalence of CD in children with T1DM in Arabs in the Middle East. The Agency for Healthcare Research and Quality (AHRQ) report,20 the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) and the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) guidelines for the diagnosis of CD,1 reported the prevalence of CD in T1DM to be 3-12%.
CD should always be diagnosed using duodenal biopsies because of the fluctuating serum levels of CD specific antibodies in patients with T1DM; therefore duodenal biopsies with the demonstration of enteropathy should always be part of CD diagnosis.1,21 The aim of the current study was to determine the prevalence of CD in a cohort of Omani children and adolescents with T1DM followed at Sultan Qaboos University Hospital (SQUH), Muscat, Oman.
Methods
A prospective cross sectional study of 103 children with T1DM followed at the pediatric diabetes clinic and seen at the pediatric gastroenterology unit, at SQUH, Muscat, Oman was carried out. The screening for CD was done for all patients referred over a period of one year (June 2011 to May 2012). Patients’ charts were reviewed for baseline demographic and clinical data. Patients were interviewed at the time of visit for symptoms compatible with CD. All patients were screened with the use of serum anti-tTGA antibodies (determined by enzyme-linked immunosorbent assay ‘ELISA’ with human recombinant tTGA as antigen, Euroimmune©). Serum EMA was also measured in 81 patients (determined by indirect immunofluorescence method) and results were reported as positive or negative. Serum IgA level was also measured to exclude IgA deficiency. The results were considered positive when anti-tTGA levels were higher than 20 U/mL. Patients with positive anti-tTGA were brought in for confirmation of CD by upper gastrointestinal endoscopy and intestinal biopsies. Multiple intestinal biopsies were taken from the second part of the duodenum (4-6 biopsies) and duodenal bulb (2 biopsies). An expert pathologist reviewed all the biopsy specimens. Histological pattern indicative of CD (Marsh 1-3 lesions) of Marsh-Oberhuber classification,22 were considered as compatible with the diagnosis of CD in the presence of positive CD antibodies. Ethical approval was obtained through the ethical committee at the College of Medicine and Health Sciences, Sultan Qaboos University.
Descriptive statistics were used to describe the data. For categorical variables, frequencies and percentages were reported. Differences between groups were analyzed using Pearson’s chi-square test (or Fisher’s exact test for cells <5). For continuous variables, means and standard deviation, as well as median and interquartile range (25th and 75th percentiles) were used to present the data while analysis was performed using Student’s t-test and Wilcoxon-Mann-Whitney, respectively. A priori two-tailed level of significance was set at the 0.05 level. Statistical analyses were conducted using STATA version 12.1 (STATA Corporation, College Station, TX).
Results
Out of 103 children with T1DM referred for CD screening during the period from June 2011 to May 2012, 10 patients were lost to follow up, hence, not screened. Ninety-three patients aged 2-17 years, therefore, underwent CD screening. The demographic and clinical characteristics are shown in Table 1.
Positive anti-tTGA was found in 16 patients (17%). Fourteen patients underwent endoscopy with duodenal biopsies, while two more patients were lost to follow-up, leaving a total of 91 patients in the studied cohort. Three more patients underwent endoscopy and duodenal biopsies due to gastrointestinal symptoms suggestive of CD despite a negative screening. There were no IgA deficient patients. Five out of the total 14 patients (positive anti-tTGA) who underwent endoscopy were proven to have CD on intestinal biopsies. All of the 3 patients with gastrointestinal symptoms and negative screening had normal biopsies (Fig. 1). This reveals a prevalence of biopsy proven CD in children with T1DM to be 5.5% (5 out of 91) in this cohort.
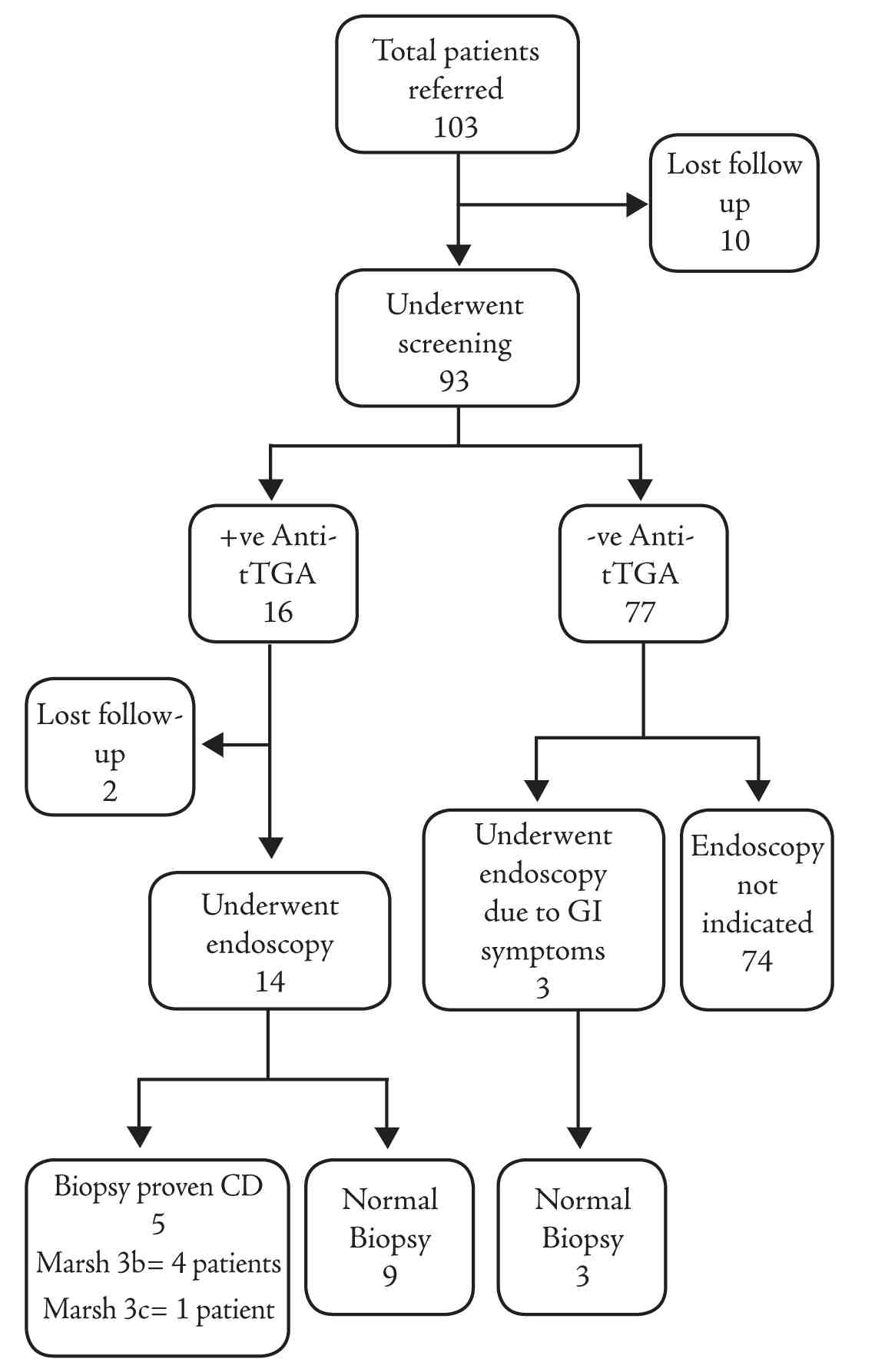
Figure 1: Flowchart for results of serologic tests and biopsy proven celiac disease.
+ve: positive, -ve: negative; Anti-tTGA: anti-tissue transglutaminase IgA; GI: gastrointestinal, CD: celiac disease.
The clinical characteristics of patients are shown in Table 1. The mean age at diagnosis of T1DM was significantly lower in patients with CD compared to those without CD (3.4 versus 6.2 years; p=0.003). Family history (FH) of T1DM representing autoimmunity was found in 60% of patients with CD versus 19% in those without CD; however, the difference was only marginally significant (p=0.06). There were no significant differences in terms of gastrointestinal symptoms (abdominal pain, distension, chronic diarrhea or constipation), anemia or duration of T1DM between the two groups. Females were slightly more prevalent in the biopsy proven CD group; however, the difference was not statistically significant (p=0.65).
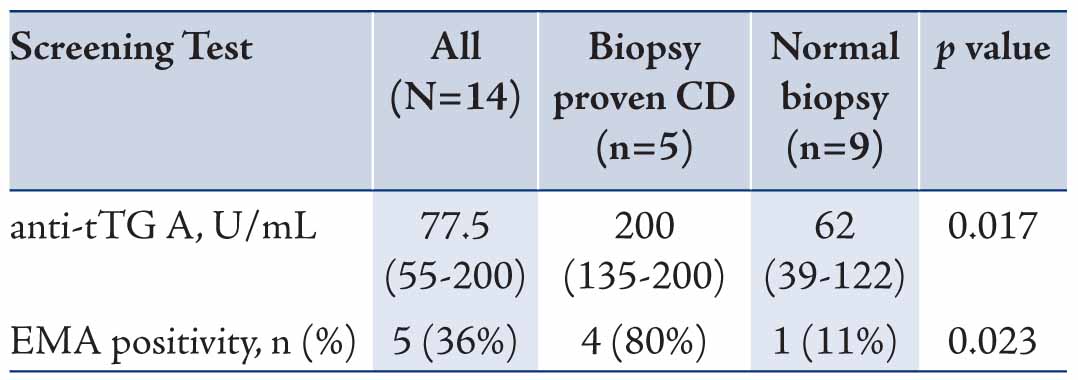
The serological and histological characteristics of patients with positive CD screening who underwent endoscopy are shown in Table 2. Higher median anti-tTGA levels were found in those with CD confirmed by intestinal biopsies compared to those with normal biopsies (200 versus 62 U/ml; p=0.017). EMA was positive in 4 out of 5 (80%) biopsy proven CD compared to 1 (11%) in patients that had normal biopsies (p=0.023). There were no patients with Marsh 1 or 2 in this cohort, Marsh classification 3b was found in 4 patients, while Marsh 3c was found in only 1 patient. (Fig. 1)
Table 1: Demographic and clinical characteristics of Omani children with type 1 diabetes mellitus (T1DM) stratified by celiac disease (CD).
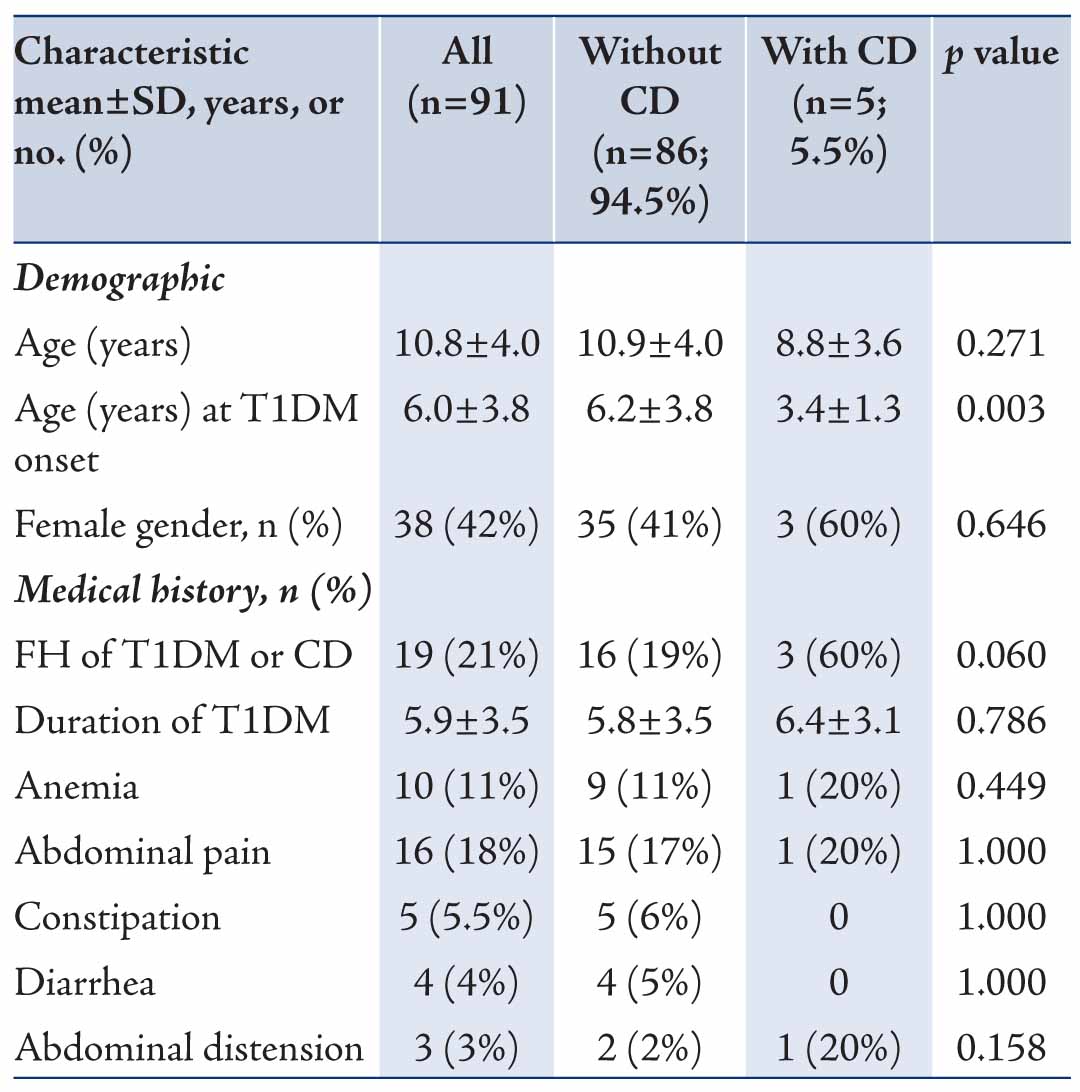
Table 2: Serological characteristics of patients with positive celiac disease (CD) auto antibodies who underwent endoscopy (n= 14). Data are presented as median (Interquartile range: IQR).
Discussion
Our study identified CD in patients with T1DM by using the diagnostic criteria recommended by the ESPGHAN. This included the combination of two highly sensitive and specific tests (anti-tTGA and EMA), and by performing 6 to 8 duodenal biopsies (including the bulb) in patients with positive serology. There are few studies conducted in the Middle East in which screening for CD-associated antibodies includes the combination of anti-tTGA and EMA was followed.6
In Oman, there is no population-based study indicating the prevalence of CD in children and adolescents. The current study is the first reported study of the prevalence of CD in Omani children and adolescents with T1DM. The prevalence of biopsy proven CD among Omani children with T1DM (5.5%) is lower than its prevalence in Middle Eastern Arabs,5,6 but is comparable to the prevalence range in North African Arabs,8-10 and the Europeans.11,12
The predominance of female gender in the CD group in our study has been demonstrated in few other studies.5,6,16,23 Our patients with T1DM and CD exhibited no differences in gastrointestinal symptoms, anemia or duration of T1DM compared to patients with T1DM without CD. On the contrary, the onset of T1DM was significantly earlier in patients with CD (p=0.003). This finding is in agreement with other studies which reported an early onset of T1DM in children with CD.11,12 This is however, different from what was reported earlier in a study of Saudi children with T1DM and CD.5,6
The results of the current study demonstrate that higher levels of anti-tTGA are positively correlated with the degree of histological changes. Higher anti-tTGA levels were found in biopsy confirmed CD patients compared with patients having normal biopsies (p=0.017). This positive correlation has also been reported in other studies.6,24,25 EMA was positive in 80% of biopsy proven CD compared to 11% in patients with normal biopsies (p=0.023). All patients reported in this study had severe form of enteropathy, which corresponds to Marsh 3b and 3c. The degree of histological changes in this study’s population may reflect the long duration of gluten exposure and correlates with their high levels of anti-tTGA levels.
The ESPGHAN guidelines recommend performing upper endoscopy and intestinal biopsies if anti-tTGA titers exceed three times the upper limit of normal in high-risk groups like T1DM patients.1 Our results support this recommendation in this high-risk group. Almost uniformly, the prevalence of CD by biopsy was lower than the prevalence by serology. This may reflect the latent nature of predisposition or low-titre serology and follow up of these patients in the form of annual screening by CD serological markers is recommended.21,26
Conclusion
The prevalence of CD in Omani children and adolescents with T1DM was 5.5%. This is the first reported study on the prevalence of CD in this age and population group. It is in keeping with the reported prevalence in the world but is less than what has been reported in a few Middle Eastern studies. Our results support the international screening recommendations in this high-risk group even in the absence of symptoms. The onset of T1DM was significantly earlier in children with CD and there was a slight predominance of female gender and positive family history of autoimmunity in the CD group. Higher anti-tTGA levels might be used as a confirmatory tool in case biopsies are not possible. These conclusions need to be solidified by a larger national level study.
Acknowledgments
The authors declare that they have no conflict of interests and no funding was received for this work.
References
1. Husby S, Koletzko S, Korponay-Szabó IR, Mearin ML, Phillips A, Shamir R, et al; ESPGHAN Working Group on Coeliac Disease Diagnosis; ESPGHAN Gastroenterology Committee; European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr 2012 Jan;54(1):136-160.
2. Mäki M, Collin P. Coeliac disease. Lancet 1997 Jun;349(9067):1755-1759.
3. Holmes GK, Prior P, Lane MR, Pope D, Allan RN. Malignancy in coeliac disease–effect of a gluten free diet. Gut 1989 Mar;30(3):333-338.
4. Chan AW, Butzner JD, McKenna R, Fritzler MJ. Tissue transglutaminase enzyme-linked immunosorbent assay as a screening test for celiac disease in pediatric patients. Pediatrics 2001 Jan;107(1):E8.
5. Saadah OI, Al-Agha AE, Al Nahdi HM, Bokhary RY, Bin Talib YY, Al-Mughales JA, et al. Prevalence of celiac disease in children with type 1 diabetes mellitus screened by anti-tissue transglutaminase antibody from Western Saudi Arabia. Saudi Med J 2012 May;33(5):541-546.
6. Al-Hussaini A, Sulaiman N, Al-Zahrani M, Alenizi A, El Haj I. High prevalence of celiac disease among Saudi children with type 1 diabetes: a prospective cross-sectional study. BMC Gastroenterol 2012;12:180.
7. Al-Ashwal AA, Shabib SM, Sakati NA, Attia NA. Prevalence and characteristics of celiac disease in type I diabetes mellitus in Saudi Arabia. Saudi Med J 2003 Oct;24(10):1113-1115.
8. Mankaï A, Ben Hamouda H, Amri F, Ghedira-Besbes L, Harbi A, Tahar Sfar M, et al. Screening by anti-endomysium antibodies for celiac disease in Tunisian children with type 1 diabetes mellitus. Gastroenterol Clin Biol 2007 May;31(5):462-466.
9. Boudraa G, Hachelaf W, Benbouabdellah M, Belkadi M, Benmansour FZ, Touhami M. Prevalence of coeliac disease in diabetic children and their first- degree relatives in west Algeria: screening with serological markers. Acta Paediatr Suppl 1996 May;412:58-60.
10. Ashabani A, Abushofa U, Abusrewill S, Abdelazez M, Tucková L, Tlaskalová-Hogenová H. The prevalence of coeliac disease in Libyan children with type 1 diabetes mellitus. Diabetes Metab Res Rev 2003 Jan-Feb;19(1):69-75.
11. Cerutti F, Bruno G, Chiarelli F, Lorini R, Meschi F, Sacchetti C; Diabetes Study Group of the Italian Society of Pediatric Endocrinology and Diabetology. Younger age at onset and sex predict celiac disease in children and adolescents with type 1 diabetes: an Italian multicenter study. Diabetes Care 2004 Jun;27(6):1294-1298.
12. Hansen D, Bennedbaek FN, Hansen LK, Høier-Madsen M, Hegedü LS, Jacobsen BB, et al. High prevalence of coeliac disease in Danish children with type I diabetes mellitus. Acta Paediatr 2001 Nov;90(11):1238-1243.
13. Aktay AN, Lee PC, Kumar V, Parton E, Wyatt DT, Werlin SL. The prevalence and clinical characteristics of celiac disease in juvenile diabetes in Wisconsin. J Pediatr Gastroenterol Nutr 2001 Oct;33(4):462-465.
14. Gillett PM, Gillett HR, Israel DM, Metzger DL, Stewart L, Chanoine JP, et al. High prevalence of celiac disease in patients with type 1 diabetes detected by antibodies to endomysium and tissue transglutaminase. Can J Gastroenterol 2001 May;15(5):297-301.
15. Hummel M, Bonifacio E, Stern M, Dittler J, Schimmel A, Ziegler AG. Development of celiac disease-associated antibodies in offspring of parents with type I diabetes. Diabetologia 2000 Aug;43(8):1005-1011.
16. Shahbazkhani B, Faezi T, Akbari MR, Mohamadnejad M, Sotoudeh M, Rajab A, et al. Coeliac disease in Iranian type I diabetic patients. Dig Liver Dis 2004 Mar;36(3):191-194.
17. Fraser JS, Woodhouse NJ, El-Shafie OT, Al-Kindy SS, Ciclitira PJ. Occult celiac disease in adult Omanis with unexplained iron deficiency anemia. Saudi Med J 2003 Jul;24(7):791.
18. Akinbami FO, Venugopalan P, Elnour IB, Nirmala V, Abiodun P, Azubuike JC. Pattern of chronic diarrhoea in children: a prospective analysis of causes, clinical features and outcome. Niger Postgrad Med J 2006 Mar;13(1):53-56.
19. Al-Lawati TT, Al-Musawi HS. Celiac disease in oman: a tertiary centre experience. Oman Med J 2013 Jan;28(1):70-72.
20. Rostom A, Dubé C, Cranney A, Saloojee N, Sy R, Garritty C, et al. Celiac disease. Evid Rep Technol Assess (Summ) 2004 Jun;104(104):1-6.
21. Vécsei A, Arenz T, Heilig G, Arenz S, Bufler P, Koletzko S. Influence of age and genetic risk on anti-tissue transglutaminase IgA titers. J Pediatr Gastroenterol Nutr 2009 May;48(5):544-549.
22. Oberhuber G, Granditsch G, Vogelsang H. The histopathology of coeliac disease: time for a standardized report scheme for pathologists. Eur J Gastroenterol Hepatol 1999 Oct;11(10):1185-1194.
23. Fallahi GH, Ahmadian JH, Rabbani A, Yousefnezhad AS, Rezaei N. Screening for celiac disease in diabetic children from Iran. Indian Pediatr 2010 Mar;47(3):268-270.
24. Dahlbom I, Korponay-Szabó IR, Kovács JB, Szalai Z, Mäki M, Hansson T. Prediction of clinical and mucosal severity of coeliac disease and dermatitis herpetiformis by quantification of IgA/IgG serum antibodies to tissue transglutaminase. J Pediatr Gastroenterol Nutr 2010 Feb;50(2):140-146.
25. Vivas S, Ruiz de Morales JG, Riestra S, Arias L, Fuentes D, Alvarez N, et al. Duodenal biopsy may be avoided when high transglutaminase antibody titers are present. World J Gastroenterol 2009 Oct;15(38):4775-4780.
26. Larsson K, Carlsson A, Cederwall E, Jönsson B, Neiderud J, Jonsson B, et al; Skåne Study Group. Annual screening detects celiac disease in children with type 1 diabetes. Pediatr Diabetes 2008 Aug;9(4 Pt 2):354-359.
|